Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism
- PMID: 20597103
- PMCID: PMC2913288
- DOI: 10.1002/emmm.201000079
Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism
Abstract
The progression of human tuberculosis (TB) to active disease and transmission involves the development of a caseous granuloma that cavitates and releases infectious Mycobacterium tuberculosis bacilli. In the current study, we exploited genome-wide microarray analysis to determine that genes for lipid sequestration and metabolism were highly expressed in caseous TB granulomas. Immunohistological analysis of these granulomas confirmed the disproportionate abundance of the proteins involved in lipid metabolism in cells surrounding the caseum; namely, adipophilin, acyl-CoA synthetase long-chain family member 1 and saposin C. Biochemical analysis of the lipid species within the caseum identified cholesterol, cholesteryl esters, triacylglycerols and lactosylceramide, which implicated low-density lipoprotein-derived lipids as the most likely source. M. tuberculosis infection in vitro induced lipid droplet formation in murine and human macrophages. Furthermore, the M. tuberculosis cell wall lipid, trehalose dimycolate, induced a strong granulomatous response in mice, which was accompanied by foam cell formation. These results provide molecular and biochemical evidence that the development of the human TB granuloma to caseation correlates with pathogen-mediated dysregulation of host lipid metabolism.
Figures


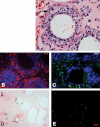
Nascent granulomas exhibit weak ADFP expression.
Caseous granulomas stain strongly for ADFP.
Fibrocaseous granulomas also label strongly for ADFP expression.
The caseous center also has intense ADFP expression, together with nuclear debris.
Resolved granulomas exhibit low levels of ADFP labeling.
Control normal lung parenchyma shows ADFP expression in pneumocytes and alveolar macrophages (the left image is a merged image with bright field). Scale bar is 50 µm.



Dual solvent separation of granuloma lipids by TLC. Total lipids were extracted from normal lung tissues (lane 4–6, 120 µg) and caseous TB lung granulomas (lane 7–9, 120 µg), and then equivalent amounts were run in parallel with total lipids from Mtb CDC1551 (lane 1, 400 µg), and standard lipids, cardiolipin (lane 2, 50 µg), phosphatidylcholine (lane 3, 50 µg), CHOL (lane 10, 20 µg), CE (lane 11, 25 µg), TAG (lane 12, 20 µg). The lipids from the TB caseum exhibit an abundance of CHOL, CE, and TAG.
Single solvent separation of lipid species with similar migration to the sphingolipids, illustrating the enrichment of LacCer in the caseum extract. Total lipids from the caseum (lane 2, 120 µg) were run together with sphingomyelin standard (lane 1, 5 µg) and neutral glycosphingolipids standard (lane 3, 30 µg). Caseous and fibrocaseous granuloma samples were derived from seven independent tissue samples, and normal lung tissue samples.
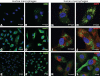
Histological examination demonstrated the extensive recruitment of cells to TDM-coated beads, many of which had the appearance of lipid droplet-filled foam cells (A representative cell is marked with an arrow).
Immunohistology on the consecutive section of the same tissue block revealed a strong expression of lipid droplet-associated protein, Adfp (nuclei in blue and Adfp in red).
Lipid droplets were detected by staining neutral lipids with Nile Red on cryosections from TDM-induced granuloma (nuclei in blue and neutral lipids in green).
In contrast, control lipid PG-coated beads demonstrated considerably reduced recruitment of cells and no induction of foam cell formation (H&E).
Moreover, probing with anti-Adfp antibody on the consecutive section cut from the same tissue block as (D) did not label any of the cells surrounding the PG-coated beads (nuclei in blue and Adfp in red). The experiments were repeated three times, and three mice were used for PG- or TDM-Matrigel mixture injection.

Intracellular Mtb bacilli synthesize and release cell wall components inside their host cells. We have demonstrated previously that these lipids accumulate in the internal vesicles in multi-vesicular bodies, which are exocytosed from the cell in vesicular form.
Because of the release of these vesicles, both infected and uninfected macrophages are exposed to cell wall mycolates and induced to form foamy macrophages, as illustrated in Fig 7. The foamy macrophages have been shown to support the maintenance and growth of persistent bacteria.
We now propose that these cells die via an inflammatory, necrotic process and release their lipid droplets into the extracellular milieu within the granuloma. As a result of the fibrotic capsule, the human granuloma is an enclosed, isolated structure with minimal vasculature. The enclosed nature of the human granuloma leads to accumulation of necrotic debris as caseum. In this model, this process is an integral part of the pathology that leads to active disease and transmission.
References
-
- Actor JK, Olsen M, Hunter RL, Jr, Geng YJ. Dysregulated response to mycobacterial cord factor trehalose-6,6′-dimycolate in CD1D−/− mice. J Interferon Cytokine Res. 2001;21:1089–1096. - PubMed
-
- Alvarez HM, Steinbuchel A. Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol. 2002;60:367–376. - PubMed
-
- Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. - PubMed
-
- Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80:31–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

