Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings
- PMID: 20597693
- PMCID: PMC2946373
- DOI: 10.1086/655143
Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings
Abstract
Background: Cryptococcal meningitis (CM) remains a common AIDS-defining illness in Africa and Asia. Subclinical cryptococcal antigenemia is frequently unmasked with antiretroviral therapy (ART). We sought to define the cost-effectiveness of serum cryptococcal antigen (CRAG) screening to identify persons with subclinical cryptococcosis and the efficacy of preemptive fluconazole therapy.
Methods: There were 609 ART-naive adults with AIDS who started ART in Kampala, Uganda, and who had a serum CRAG prospectively measured during 2004-2006. The number needed to test and treat with a positive CRAG was assessed for > or = 30-month outcomes.
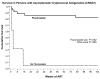
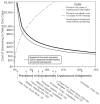
Results: In the overall cohort, 50 persons (8.2%) were serum CRAG positive when starting ART. Of 295 people with a CD4(+) cell count < or = 100 cells/microL and without prior CM, 26 (8.8%; 95% confidence interval [CI], 5.8%-12.6%) were CRAG positive, of whom 21 were promptly treated with fluconazole (200-400 mg) for 2-4 weeks. Clinical CM developed in 3 fluconazole-treated persons, and 30-month survival was 71% (95% CI, 48%-89%). In the 5 CRAG-positive persons with a CD4(+) cell count < or = 100 cells/microL treated with ART but not fluconazole, all died within 2 months of ART initiation. The number needed to test and treat with CRAG screening and fluconazole to prevent 1 CM case is 11.3 (95% CI, 7.9-17.1) at costs of $190 (95% CI, $132-$287). The number needed to test and treat to save 1 life is 15.9 (95% CI, 11.1-24.0) at costs of $266 (95% CI, $185-$402). The cost per disability-adjusted life year saved is $21 (95% CI, $15-$32).
Conclusions: Integrating CRAG screening into HIV care, specifically targeting people with severe immunosuppression (CD4(+) cell count < or = 100 cells/microL) should be implemented in treatment programs in resource-limited settings. ART alone is insufficient treatment for CRAG-positive persons.
Conflict of interest statement
Pfizer manufactures and donates fluconazole for use in Sub-Saharan Africa via the Pfizer Diflucan Partnership program. Pfizer had no role in any aspect of this project.
The authors have no potential conflicts of interest.
Figures

Comment in
-
Cryptococcal antigen screening for patients initiating antiretroviral therapy: time for action.Clin Infect Dis. 2010 Dec 15;51(12):1463-5. doi: 10.1086/657405. Clin Infect Dis. 2010. PMID: 21082878 No abstract available.
References
-
- Park BJ, Wannwmuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. - PubMed
-
- Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998;26:1362–6. - PubMed
-
- Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

