Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency
- PMID: 20597972
- PMCID: PMC4454453
- DOI: 10.1111/j.1460-9568.2010.07244.x
Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency
Abstract
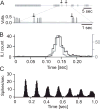
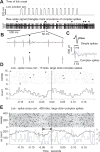
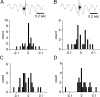
Rodents consume water by performing stereotypic, rhythmic licking movements that are believed to be controlled by brainstem pattern-generating circuits. Previous work has shown that synchronized population activity of inferior olive neurons was phase-locked to the licking rhythm in rats, suggesting a cerebellar involvement in temporal aspects of licking behavior. However, what role the cerebellum has in licking behavior and whether licking is represented in the high-frequency simple spike output of Purkinje cells remains unknown. We recorded Purkinje cell simple and complex spike activity in awake mice during licking, and determined the behavioral consequences of loss of cerebellar function. Mouse cerebellar cortex contained a multifaceted representation of licking behavior encoded in the simple spike activities of Purkinje cells distributed across Crus I, Crus II and lobus simplex of the right cerebellar hemisphere. Lick-related Purkinje cell simple spike activity was modulated rhythmically, phase-locked to the lick rhythm, or non-rhythmically. A subpopulation of lick-related Purkinje cells differentially represented lick interval duration in their simple spike activity. Surgical removal of the cerebellum or temporary pharmacological inactivation of the cerebellar nuclei significantly slowed the licking frequency. Fluid licking was also less efficient in mice with impaired cerebellar function, indicated by a significant decline in the volume per lick fluid intake. The gross licking movement appeared unaffected. Our results suggest a cerebellar role in modulating the frequency of the central pattern-generating circuits controlling fluid licking and in the fine coordination of licking, while contributing little to the coordination of the gross licking movement.
Figures



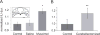
References
-
- Albus JS. A theory of cerebellar function. Mathematical Biosci. 1971;10:25–61.
-
- Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res. 1983;286:299–322. - PubMed
-
- Boughter JD, Baird JD, Bryant JL, St John SJ, Heck DH. C57BL/6J and DBA/2J Mice Vary in Lick rate and Ingestive Microstructure. Genes, Brain and Behavior. 2007;6:619–627. - PubMed
-
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

