Stem cells and cancer of the stomach and intestine
- PMID: 20598659
- PMCID: PMC5527927
- DOI: 10.1016/j.molonc.2010.05.001
Stem cells and cancer of the stomach and intestine
Abstract
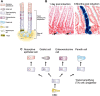
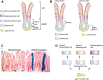
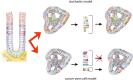
Cancer in the 21st century has become the number one cause of death in developed countries. Although much progress has been made in improving patient survival, tumour relapse is one of the important causes of cancer treatment failure. An early observation in the study of cancer was the heterogeneity of tumours. Traditionally, this was explained by a combination of genomic instability of tumours and micro environmental factors leading to diverse phenotypical characteristics. It was assumed that cells in a tumour have an equal capacity to propagate the cancer. This model is currently known as the stochastic model. Recently, the Cancer stem cell model has been proposed to explain the heterogeneity of a tumour and its progression. According to this model, the heterogeneity of tumours is the result of aberrant differentiation of tumour cells into the cells of the tissue the tumour originated from. Tumours were suggested to contain stem cell-like cells, the cancer stem cells or tumour-initiating cells, which are uniquely capable of propagating a tumour much like normal stem cells fuel proliferation and differentiation in normal tissue. In this review we discuss the normal stem cell biology of the stomach and intestine followed by both the stochastic and cancer stem cell models in light of recent findings in the gastric and intestinal systems. The molecular pathways underlying normal and tumourigenic growth have been well studied, and recently the stem cells of the stomach and intestine have been identified. Furthermore, intestinal stem cells were identified as the cells-of-origin of colon cancer upon loss of the tumour suppressor APC. Lastly, several studies have proposed the positive identification of a cancer stem cell of human colon cancer. At the end we compare the cancer stem cell model and the stochastic model. We conclude that clonal evolution of tumour cells resulting from genetic mutations underlies tumour initiation and progression in both cancer models. This implies that at any point during tumour development any tumour cell can revert to a cancer stem cell after having gained a clonal advantage over the original cancer stem cell. Therefore, these models represent two sides of the same coin.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Barker, N. , Huch, M. , Kujala, P. , van de Wetering, M. , Snippert, H.J. , van Es, J.H. , Sato, T. , Stange, D.E. , Begthel, H. , van den Born, M. , Danenberg, E. , van den Brink, S. , Korving, J. , Abo, A. , Peters, P.J. , Wright, N. , Poulsom, R. , Clevers, H. , 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6, 25–36. - PubMed
-
- Barker, N. , Ridgway, R.A. , van Es, J.H. , van de Wetering, M. , Begthel, H. , van den Born, M. , Danenberg, E. , Clarke, A.R. , Sansom, O.J. , Clevers, H. , 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 457, 608–611. - PubMed
-
- Barker, N. , van Es, J.H. , Kuipers, J. , Kujala, P. , van den Born, M. , Cozijnsen, M. , Haegebarth, A. , Korving, J. , Begthel, H. , Peters, P.J. , Clevers, H. , 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449, 1003–1007. - PubMed
-
- Batlle, E. , Bacani, J. , Begthel, H. , Jonkheer, S. , Gregorieff, A. , van de Born, M. , Malats, N. , Sancho, E. , Boon, E. , Pawson, T. , Gallinger, S. , Pals, S. , Clevers, H. , 2005. EphB receptor activity suppresses colorectal cancer progression. Nature. 435, 1126–1130. - PubMed
-
- Batlle, E. , Henderson, J.T. , Beghtel, H. , van den Born, M.M. , Sancho, E. , Huls, G. , Meeldijk, J. , Robertson, J. , van de Wetering, M. , Pawson, T. , Clevers, H. , 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 111, 251–263. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

