Intrinsically disordered proteins are potential drug targets
- PMID: 20598937
- PMCID: PMC2918680
- DOI: 10.1016/j.cbpa.2010.06.169
Intrinsically disordered proteins are potential drug targets
Abstract
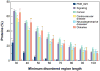
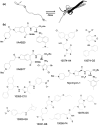
Intrinsically disordered (ID) proteins that lack stable secondary and tertiary structure in substantial regions (or throughout) are prevalent in eukaryotes. They exist as ensembles of rapidly fluctuating structures and many undergo coupled folding and binding reactions. Because ID proteins are overrepresented in major disease pathways they are desirable targets for inhibition; however, the feasibility of targeting proteins without defined structures was unclear. Recently, small molecules have been found that bind to the disordered regions of c-Myc, Abeta, EWS-Fli1, and various peptides. As with structured targets, initial hits were further optimized to increase specificity and affinity. Given the number and biological importance of ID proteins, the ability to inhibit their interactions opens tremendous potential in chemical biology and drug discovery.
2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5:821–834. - PubMed
-
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. - PubMed
-
- Yin H, Hamilton AD. Strategies for targeting protein-protein interactions with synthetic agents. Angew Chem-Int Edit. 2005;44:4130–4163. - PubMed
-
- Clackson T, Wells JA. A Hot-Spot of Binding-Energy in a Hormone-Receptor Interface. Science. 1995;267:383–386. - PubMed
-
- Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18:756–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

