Intestinal stem cells and their roles during mucosal injury and repair
- PMID: 20599211
- PMCID: PMC4040212
- DOI: 10.1016/j.jss.2010.04.037
Intestinal stem cells and their roles during mucosal injury and repair
Abstract
The ability of the host to respond to intestinal injury requires the regeneration of native tissue through a highly orchestrated response from the intestinal stem cells, a population of cells located within the intestinal crypts that have the capability to repopulate the entire villous. The field of intestinal stem cell biology is thus of great interest to surgeons and non-surgeons alike, given its relevance to diseases of intestinal injury and inflammation such as inflammatory bowel disease, trauma, and necrotizing enterocolitis. The field of intestinal stem cell research has been advanced recently by the identification of the putative marker, Lgr5, which has allowed for the isolation and further characterization of the intestinal stem cell. Under the control of the WNT signaling pathway, Lgr5 marks the rapidly dividing cells of the intestinal crypt, and identifies a population of cells that is capable of regenerating the entire villous. We now review the identification of Lgr5 as an intestinal stem cell marker, identify controversies in the intestinal stem cell field, and highlight the response of the intestinal stem cell to injury within the intestinal mucosa that may occur clinically.
Copyright © 2011. Published by Elsevier Inc.
Figures
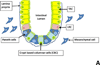

Similar articles
-
Intestinal stem cells and inflammation.Curr Opin Pharmacol. 2015 Dec;25:62-6. doi: 10.1016/j.coph.2015.11.008. Epub 2015 Dec 2. Curr Opin Pharmacol. 2015. PMID: 26654865 Review.
-
Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium.Gut. 2014 Apr;63(4):610-21. doi: 10.1136/gutjnl-2012-304067. Epub 2013 Jul 5. Gut. 2014. PMID: 23831735 Free PMC article.
-
Wnt signaling, lgr5, and stem cells in the intestine and skin.Am J Pathol. 2009 Mar;174(3):715-21. doi: 10.2353/ajpath.2009.080758. Epub 2009 Feb 5. Am J Pathol. 2009. PMID: 19197002 Free PMC article. Review.
-
Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury.Science. 2012 Oct 5;338(6103):108-13. doi: 10.1126/science.1223821. Epub 2012 Sep 6. Science. 2012. PMID: 22956684 Free PMC article.
-
Heterogeneity in readouts of canonical wnt pathway activity within intestinal crypts.Dev Dyn. 2016 Aug;245(8):822-33. doi: 10.1002/dvdy.24423. Epub 2016 Jul 5. Dev Dyn. 2016. PMID: 27264700 Free PMC article.
Cited by
-
Innate immune signaling in the pathogenesis of necrotizing enterocolitis.Clin Dev Immunol. 2013;2013:475415. doi: 10.1155/2013/475415. Epub 2013 May 23. Clin Dev Immunol. 2013. PMID: 23762089 Free PMC article. Review.
-
Intestinal tuft cells regulate the ATM mediated DNA Damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury.Sci Rep. 2016 Nov 23;6:37667. doi: 10.1038/srep37667. Sci Rep. 2016. PMID: 27876863 Free PMC article.
-
Influence of stress factors on intestinal epithelial injury and regeneration.Pediatr Surg Int. 2018 Feb;34(2):155-160. doi: 10.1007/s00383-017-4183-3. Epub 2017 Oct 10. Pediatr Surg Int. 2018. PMID: 29018960
-
Assessment of the mode of action underlying development of rodent small intestinal tumors following oral exposure to hexavalent chromium and relevance to humans.Crit Rev Toxicol. 2013 Mar;43(3):244-74. doi: 10.3109/10408444.2013.768596. Crit Rev Toxicol. 2013. PMID: 23445218 Free PMC article. Review.
-
Clinical and histopathological correlates of intestinal repair in preterm infants following surgical necrotizing enterocolitis.J Matern Fetal Neonatal Med. 2022 Dec;35(26):10565-10576. doi: 10.1080/14767058.2022.2134773. Epub 2022 Oct 19. J Matern Fetal Neonatal Med. 2022. PMID: 36261134 Free PMC article.
References
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. - PubMed
-
- Van der Flier, LG and Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009;71:241–260. - PubMed
-
- Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002;115:2381–2388. - PubMed
-
- Batlle E. A new identity for the elusive stem cell. Nat Genet. 2008 Jul;40(7):818–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

