Circadian clocks in the ovary
- PMID: 20599392
- PMCID: PMC2949464
- DOI: 10.1016/j.tem.2010.06.002
Circadian clocks in the ovary
Abstract
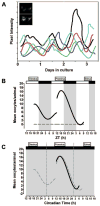
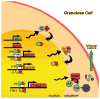
Clock gene expression has been observed in tissues of the hypothalamic-pituitary-gonadal (HPG) axis. Whereas the contribution of hypothalamic oscillators to the timing of reproductive biology is well known, the role of peripheral oscillators like those in the ovary is less clear. Circadian clocks in the ovary might play a role in the timing of ovulation. Disruption of the clock in ovarian cells or desynchrony between ovarian clocks and circadian oscillators elsewhere in the body may contribute to the onset and progression of various reproductive pathologies. In this paper, we review evidence for clock function in the ovary across a number of species and offer a novel perspective into the role of this clock in normal ovarian physiology and in diseases that negatively affect fertility.
Published by Elsevier Ltd.
Figures

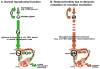
References
-
- Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17:141. - PubMed
-
- Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod. 2003;69:81–91. - PubMed
-
- Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. - PubMed
-
- Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

