Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters
- PMID: 20599443
- PMCID: PMC7112678
- DOI: 10.1016/j.pharmthera.2010.06.003
Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters
Abstract
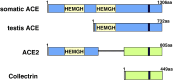
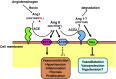
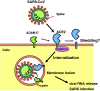
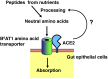
Angiotensin-converting enzyme (ACE) 2 is a homolog to the carboxypeptidase ACE, which generates angiotensin II, the main active peptide of renin-angiotensin system (RAS). After the cloning of ACE2 in 2000, three major ACE2 functions have been described so far. First ACE2 has emerged as a potent negative regulator of the RAS counterbalancing the multiple functions of ACE. By targeting angiotensin II ACE2 exhibits a protective role in the cardiovascular system and many other organs. Second ACE2 was identified as an essential receptor for the SARS coronavirus that causes severe acute lung failure. Downregulation of ACE2 strongly contributes to the pathogenesis of severe lung failure. Third, both ACE2 and its homologue Collectrin can associate with amino acid transporters and play essential role in the absorption of amino acids in the kidney and gut. In this review, we will discuss the multiple biological functions of ACE2.
2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Akpinar P., Kuwajima S., Krutzfeldt J., Stoffel M. Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab. 2005;2:385–397. - PubMed
-
- Alenina N., Xu P., Rentzsch B., Patkin E.L., Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. - PubMed
-
- Baron D.N., Dent C.E., Harris H., Hart E.W., Jepson J.B. Hereditary pellagra-like skin rash with temporary cerebellar ataxia, constant renal amino-aciduria, and other bizarre biochemical features. Lancet. 1956;271:421–428. - PubMed
-
- Belova L.A. Angiotensin II-generating enzymes. Biochemistry (Mosc) 2000;65:1337–1345. - PubMed
-
- Blau D.M., Holmes K.V. Human coronavirus HCoV-229E enters susceptible cells via the endocytic pathway. Adv Exp Med Biol. 2001;494:193–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

