Targeting leukemic stem cells by breaking their dormancy
- PMID: 20599449
- PMCID: PMC5527930
- DOI: 10.1016/j.molonc.2010.06.001
Targeting leukemic stem cells by breaking their dormancy
Abstract
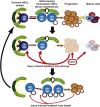
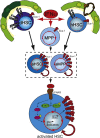
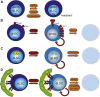
Transient or long-term quiescence, the latter referred to as dormancy are fundamental features of at least some adult stem cells. The status of dormancy is likely a critical mechanism for the observed resistance of normal HSCs and leukemic stem cells (LSCs) to anti-proliferative chemotherapy. Recent studies have revealed cytokines such as Interferon-alpha (IFNα) and G-CSF as well as arsenic trioxide (As(2)O(3)) to be efficient agents for promoting cycling of dormant HSCs and LSCs. Most interestingly, such cell cycle activated stem cells become exquisitely sensitive to killing by different chemotherapeutic agents, suggesting that dormant LSCs in patients may be targeted by a sequential two-step protocol involving an initial activation by IFNα, G-CSF or As(2)O(3), followed by targeted chemotherapy.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Awakening dormant haematopoietic stem cells.Nat Rev Immunol. 2010 Mar;10(3):201-9. doi: 10.1038/nri2726. Nat Rev Immunol. 2010. PMID: 20182459
-
Differential expression and response to arsenic stress of MRPs and ASAN1 determine sensitivity of classical multidrug-resistant leukemia cells to arsenic trioxide.Leuk Res. 2016 Nov;50:116-122. doi: 10.1016/j.leukres.2016.10.003. Epub 2016 Oct 3. Leuk Res. 2016. PMID: 27736728
-
Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses.J Biol Chem. 2008 Jan 25;283(4):1992-2001. doi: 10.1074/jbc.M705227200. Epub 2007 Nov 29. J Biol Chem. 2008. PMID: 18048359
-
In vivo and in vitro treatment of HTLV-1 and HTLV-2 infected cells with arsenic trioxide and interferon-alpha.Leuk Lymphoma. 2005 Mar;46(3):347-55. doi: 10.1080/10428190400019966. Leuk Lymphoma. 2005. PMID: 15621824 Review.
-
Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: an useful salvage therapy.Leuk Lymphoma. 2000 Jul;38(3-4):283-93. doi: 10.3109/10428190009087019. Leuk Lymphoma. 2000. PMID: 10830735 Review.
Cited by
-
Stromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasms.Blood Cancer J. 2012 Sep 7;2(9):e87. doi: 10.1038/bcj.2012.36. Blood Cancer J. 2012. PMID: 22961059 Free PMC article.
-
Systematic Classification of Mixed-Lineage Leukemia Fusion Partners Predicts Additional Cancer Pathways.Ann Lab Med. 2016 Mar;36(2):85-100. doi: 10.3343/alm.2016.36.2.85. Ann Lab Med. 2016. PMID: 26709255 Free PMC article. Review.
-
Curcumin derivative C212 inhibits Hsp90 and eliminates both growing and quiescent leukemia cells in deep dormancy.Cell Commun Signal. 2020 Sep 29;18(1):159. doi: 10.1186/s12964-020-00652-4. Cell Commun Signal. 2020. PMID: 32993709 Free PMC article.
-
Analysis of marker-defined HNSCC subpopulations reveals a dynamic regulation of tumor initiating properties.PLoS One. 2012;7(1):e29974. doi: 10.1371/journal.pone.0029974. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276135 Free PMC article.
-
GLI2 inhibition abrogates human leukemia stem cell dormancy.J Transl Med. 2015 Mar 21;13:98. doi: 10.1186/s12967-015-0453-9. J Transl Med. 2015. PMID: 25889765 Free PMC article.
References
-
- Cho, R.W. , Clarke, M.F. , 2008. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 18, 48–53. - PubMed
-
- de Veer, M.J. , Holko, M. , Frevel, M. , Walker, E. , Der, S. , Paranjape, J.M. , Silverman, R.H. , Williams, B.R. , 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69, 912–920. - PubMed
-
- Dick, J.E. , 2008. Stem cell concepts renew cancer research. Blood. 112, 4793–4807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

