Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2
- PMID: 20599524
- PMCID: PMC2970630
- DOI: 10.1016/j.hrthm.2010.06.032
Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2
Abstract
Background: Alternans of intracellular Ca(2+) (Ca(i)) underlies T-wave alternans, a predictor of cardiac arrhythmias. A related phenomenon, T-wave lability (TWL), precedes torsades de pointes (TdP) in patients and animal models with impaired repolarization. However, the role of Ca(i) in TWL remains unexplored.
Objective: This study investigated the role of Ca(i) dynamics on TWL in a noncryoablated rabbit model of long QT syndrome type 2 (LQT2) using simultaneous measurements of Ca(i) transient (CaT), action potentials (APs), and electrocardiogram (ECG) during paced rhythms and focused on events that precede ventricular ectopy.
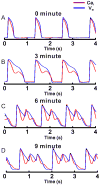
Methods: APs and CaTs were mapped optically from paced Langendorff female rabbit hearts (n = 8) at 1.2-s cycle length, after atrioventricular node ablation. Hearts were perfused with normal Tyrode solution, then with dofetilide (0.5 μM), and reduced [K(+)] (2 mM) and [Mg(2+)] (0.5 mM) to elicit LQT2. Lability of ECG, voltage, and Ca(i) signals were evaluated during regular paced rhythm, before and after dofetilide perfusion.
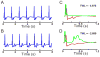
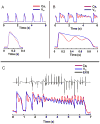
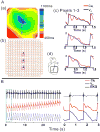
Results: In LQT2, lability of Ca(i), voltage, and ECG signals increased during paced rhythm, before the appearance of early afterdepolarizations (EADs). LQT2 resulted in AP prolongation and multiple (1 to 3) additional Ca(i) upstrokes, whereas APs remained monophasic. When EADs appeared, Ca(i) rose before voltage upstrokes at the origins of propagating EADs. Interventions (i.e., ryanodine and thapsigargin, n = 3 or low [Ca](o) and nifedipine, n = 4) that suppressed Ca(i) oscillations also abolished EADs.
Conclusion: In LQT2, Ca(i) oscillations (Ca(i)O) precede EADs by minutes, indicating that they result from spontaneous sarcoplasmic reticulum Ca(2+) release rather than spontaneous I(Ca,L) reactivation. Ca(i)O likely produce oscillations of Na/Ca exchange current, I(NCX). Depolarizing I(NCX) during the AP plateau contributes to the generation of EADs by reactivating Ca(2+) channels that have recovered from inactivation. TWL reflects CaTs and APs lability that occur before EADs and TdP.
Copyright © 2010 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Repolarization variability and early afterdepolarizations in long QT syndrome type 2: is labile calcium the common denominator?Heart Rhythm. 2010 Nov;7(11):1695-6. doi: 10.1016/j.hrthm.2010.07.007. Epub 2010 Aug 12. Heart Rhythm. 2010. PMID: 20624482 No abstract available.
References
-
- Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. The Journal of the American College of Cardiology. 2000;36:1749–1766. - PubMed
-
- Hinterseer M, Beckmann BM, Thomsen MB, et al. Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am J Cardiol. 2009;103:1244–1248. - PubMed
-
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–763. - PubMed
-
- January CT, Fozzard HA. Delayed afterdepolarizations in heart muscle: mechanisms and relevance. Pharmacological Reviews. 1988;40:219–227. - PubMed
-
- Volders PG, Vos MA, Szabo B, et al. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovascular Research. 2000;46:376–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

