Calcium indicators and calcium signalling in skeletal muscle fibres during excitation-contraction coupling
- PMID: 20599552
- PMCID: PMC2974769
- DOI: 10.1016/j.pbiomolbio.2010.06.001
Calcium indicators and calcium signalling in skeletal muscle fibres during excitation-contraction coupling
Abstract
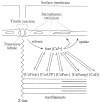
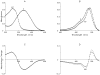
During excitation-contraction coupling in skeletal muscle, calcium ions are released into the myoplasm by the sarcoplasmic reticulum (SR) in response to depolarization of the fibre's exterior membranes. Ca(2+) then diffuses to the thin filaments, where Ca(2+) binds to the Ca(2+) regulatory sites on troponin to activate muscle contraction. Quantitative studies of these events in intact muscle preparations have relied heavily on Ca(2+)-indicator dyes to measure the change in the spatially-averaged myoplasmic free Ca(2+) concentration (Δ[Ca(2+)]) that results from the release of SR Ca(2+). In normal fibres stimulated by an action potential, Δ[Ca(2+)] is large and brief, requiring that an accurate measurement of Δ[Ca(2+)] be made with a low-affinity rapidly-responding indicator. Some low-affinity Ca(2+) indicators monitor Δ[Ca(2+)] much more accurately than others, however, as reviewed here in measurements in frog twitch fibres with sixteen low-affinity indicators. This article also examines measurements and simulations of Δ[Ca(2+)] in mouse fast-twitch fibres. The simulations use a multi-compartment model of the sarcomere that takes into account Ca(2+)'s release from the SR, its diffusion and binding within the myoplasm, and its re-sequestration by the SR Ca(2+) pump. The simulations are quantitatively consistent with the measurements and appear to provide a satisfactory picture of the underlying Ca(2+) movements.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

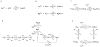
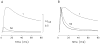

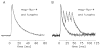
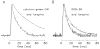
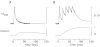

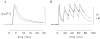
References
-
- Baylor SM, Quinta-Ferreira ME, Hui CS. Isotropic components of antipyrylazo III signals from frog skeletal muscle fibers. In: Rubin RP, Weiss G, Putney JW Jr, editors. Calcium and Biological Systems. Plenum Publishing Corp; New York: 1985. pp. 339–349.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

