The potent synthetic androgens, dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone, do not require 5α-reduction to exert their maximal androgenic effects
- PMID: 20599615
- PMCID: PMC2949447
- DOI: 10.1016/j.jsbmb.2010.06.009
The potent synthetic androgens, dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone, do not require 5α-reduction to exert their maximal androgenic effects
Abstract
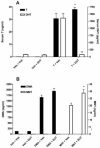
Dimethandrolone (DMA: 7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone (MNT) are potent androgens in development for hormonal therapy in men. As 5α-reduced androgens, such as 5α-dihydrotestosterone (DHT), may raise the risk of benign prostate hyperplasia, accelerate the development of prostate carcinoma, and increase male pattern baldness and acne, we investigated the role of 5α-reduction in the androgenic activity of DMA and MNT. The authentic 5α-reduced metabolites, 5α-dihydroDMA (5α-DHDMA) and 5α-dihydroMNT (5α-DHMNT), were prepared by chemical synthesis and compared in vitro and in vivo to the parent compounds. Both 5α-reduced androgens bound with high affinity to the rat androgen receptor (AR) and were potent inducers of transactivation of 3XHRE-LUC in CV-1 cells cotransfected with a human AR expression plasmid. To examine in vivo androgenic (stimulation of ventral prostate [VP] and seminal vesicle [SV] weights) and anabolic (stimulation of levator ani [LA] muscle weights) activity, 22-day-old castrate male rats were treated sc for 7 days with various doses of DMA, 5α-DHDMA, or testosterone (T) or MNT, 5α-DHMNT, or T and necropsied on day 8. 5α-DHDMA was at least threefold more potent than T in stimulating growth of the VP but only 30-40% as potent as DMA. 5α-DHMNT was four- to eightfold more potent than T, whereas MNT was approximately equipotent to T. To assess the possible role of 5α-reduction in VP and SV growth, castrate immature rats were treated with maximally effective doses of T, DHT, DMA, MNT, or the related 19-norandrogen, 7α-methyl-19-nortestosterone (MENT), or vehicle, with or without dutasteride (DUT), an inhibitor of 5α-reductases types 1 and 2. In rats treated with T+DUT, serum T was significantly higher (P<0.05) than in rats treated with T alone, and serum DHT was decreased (P<0.001) to levels observed in castrate vehicle-treated rats. DUT significantly reduced both VP and SV weights in T-treated rats, whereas there was no significant effect of DUT on weights of these accessory sex glands in rats treated with DMA, MNT, DHT, or MENT. These results indicate that inhibition of 5α-reductase activity in vivo does not affect the androgenic potency of DMA, MNT, or MENT.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures





References
-
- Sundaram K, Kumar N, Monder C, Bardin CW. Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J. Steroid Biochem. Mol. Biol. 1995;53:253–257. - PubMed
-
- Byrne MM, Nieschlag E. Testosterone replacement therapy in male hypogonadism. J Endocrinol. Invest. 2003;26:481–489. - PubMed
-
- Oettel M. The endocrine pharmacology of testosterone therapy in men. Naturwissenschaften. 2004;91:66–76. - PubMed
-
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Rev. 1994;15:342–355. - PubMed
-
- Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;14:3016–3026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

