Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity
- PMID: 20599768
- PMCID: PMC2974004
- DOI: 10.1016/j.bcp.2010.06.042
Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity
Abstract
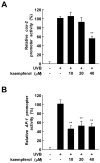
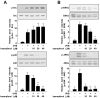
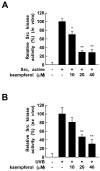
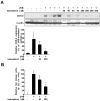
Ultraviolet (UV) radiation is the primary environmental risk factor in the development of nonmelanoma skin cancer, and UVB in particular promotes tumor growth through various signaling pathways. Kaempferol, a flavonoid with anti-inflammatory and anti-oxidative properties, has been studied as a chemopreventive agent; however, little is known regarding its effects on UVB-induced photo-carcinogenesis. Here, we examined the effect of kaempferol on UVB-induced skin inflammation. We found that kaempferol suppressed UVB-induced cyclooxygenase-2 (COX-2) protein expression in mouse skin epidermal JB6 P+ cells and attenuated the UVB-induced transcriptional activities of cox-2 and activator protein-1 (AP-1). Kaempferol attenuated the UVB-induced phosphorylation of several mitogen-activated protein kinases (MAPKs), including ERKs, p38, and JNKs, but had no effect on the phosphorylation of the upstream MAPK regulator Src. However, in vitro and ex vivo kinase assays demonstrated that kaempferol suppressed Src kinase activity. Furthermore, in vivo data from mouse skin support the idea that kaempferol suppresses UVB-induced COX-2 expression by blocking Src kinase activity. A pull-down assay revealed that kaempferol competes with ATP for direct binding to Src. Docking data suggest that kaempferol docks easily into the ATP-binding site of Src, which is located between the N and the C lobes of the kinase domain. Taken together, these results suggest that kaempferol is a potent chemopreventive agent against skin cancer through its inhibitory interaction with Src.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression.Carcinogenesis. 2009 Feb;30(2):321-30. doi: 10.1093/carcin/bgn282. Epub 2008 Dec 10. Carcinogenesis. 2009. PMID: 19073879 Free PMC article.
-
Src kinase is a direct target of apigenin against UVB-induced skin inflammation.Carcinogenesis. 2013 Feb;34(2):397-405. doi: 10.1093/carcin/bgs358. Epub 2012 Nov 17. Carcinogenesis. 2013. PMID: 23161610
-
Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer.Cancer Res. 2010 Mar 15;70(6):2415-23. doi: 10.1158/0008-5472.CAN-09-4093. Epub 2010 Mar 9. Cancer Res. 2010. PMID: 20215519
-
Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets.Arch Biochem Biophys. 2014 Oct 1;559:38-45. doi: 10.1016/j.abb.2014.05.016. Epub 2014 May 26. Arch Biochem Biophys. 2014. PMID: 24875145
-
Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets.Biochem Pharmacol. 2006 Nov 30;72(11):1506-15. doi: 10.1016/j.bcp.2006.08.005. Epub 2006 Sep 26. Biochem Pharmacol. 2006. PMID: 16999939 Review.
Cited by
-
Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability.Int J Nanomedicine. 2012;7:3951-9. doi: 10.2147/IJN.S33670. Epub 2012 Jul 24. Int J Nanomedicine. 2012. PMID: 22866004 Free PMC article.
-
Bioflavonoid exerts analgesic and anti-inflammatory effects via transient receptor potential 1 channel in a rat model.Arq Neuropsiquiatr. 2022 Sep;80(9):900-907. doi: 10.1055/s-0042-1755321. Epub 2022 Nov 9. Arq Neuropsiquiatr. 2022. PMID: 36351417 Free PMC article.
-
Flavonols in the Prevention of Diabetes-induced Vascular Dysfunction.J Cardiovasc Pharmacol. 2015 Jun;65(6):532-44. doi: 10.1097/FJC.0000000000000180. J Cardiovasc Pharmacol. 2015. PMID: 25387248 Free PMC article. Review.
-
Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis.Cell Death Dis. 2018 Mar 14;9(3):401. doi: 10.1038/s41419-018-0433-0. Cell Death Dis. 2018. PMID: 29540697 Free PMC article.
-
c-Src activation as a potential marker of chemical-induced skin irritation using tissue-engineered skin equivalents.Exp Dermatol. 2023 Feb;32(2):220-225. doi: 10.1111/exd.14719. Epub 2022 Dec 11. Exp Dermatol. 2023. PMID: 36457227 Free PMC article.
References
-
- Fears TR, Scotto J. Estimating increases in skin cancer morbidity due to increases in ultraviolet radiation exposure. Cancer Invest. 1983;1:119–26. - PubMed
-
- Strickland PT, Vitasa BC, West SK, Rosenthal FS, Emmett EA, Taylor HR. Quantitative carcinogenesis in man: solar ultraviolet B dose dependence of skin cancer in Maryland watermen. J Natl Cancer Inst. 1989;81:1910–3. - PubMed
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat Res. 1999;428:5–10. - PubMed
-
- Fischer SM. Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol. 2002;21:183–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

