Anti-cancer gallotannin penta-O-galloyl-beta-D-glucose is a nanomolar inhibitor of select mammalian DNA polymerases
- PMID: 20599777
- PMCID: PMC2943143
- DOI: 10.1016/j.bcp.2010.06.031
Anti-cancer gallotannin penta-O-galloyl-beta-D-glucose is a nanomolar inhibitor of select mammalian DNA polymerases
Abstract
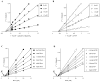
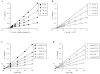
Penta-1,2,3,4,6-O-galloyl-beta-D-glucose (PGG) has been shown by us and others to inhibit the in vivo growth of human prostate cancer (PCa) xenografts in athymic nude mice and mouse lung cancer allograft in syngenic mice without evident adverse effect on their body weight. We observed a rapid inhibition of DNA synthesis in S-phase cells in PGG-exposed cancer cells and in PGG-treated isolated nuclei. The purpose of the present study was to test the hypothesis that PGG inhibits DNA replicative synthesis through a direct inhibition of one or more DNA polymerases (pols). Using purified pols, we show that PGG exhibited a selective inhibition against the activities of B-family replicative pols (alpha, delta and epsilon) and Y-family (eta, iota and kappa) of bypass synthesis pols, and the inhibitory effect of PGG on pol alpha was the strongest with IC(50) value of 13 nM. PGG also inhibited pol beta, but the potency was an order of magnitude less than against pol alpha. PGG inhibition of pol alpha and kappa activity was non-competitive with respect to the DNA template-primer and the dNTP substrate; whereas it inhibited pol beta competitively. Docking simulation on pol beta, which is the only mammalian pol with solved crystal structure, suggests several favorable interactions with the catalytic pocket/binding site for the incoming dNTP. These results support PGG as a novel inhibitor of select families of mammalian pols by distinct mechanisms, and suggest that the potent pol inhibition may contribute to its anti-cancer efficacy.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


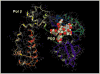
Similar articles
-
Biological and biomedical functions of Penta-O-galloyl-D-glucose and its derivatives.J Nat Med. 2014 Jul;68(3):465-72. doi: 10.1007/s11418-014-0823-2. Epub 2014 Feb 15. J Nat Med. 2014. PMID: 24532420 Review.
-
Penta-O-galloyl-beta-D-glucose induces S- and G(1)-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin D1.Carcinogenesis. 2009 May;30(5):818-23. doi: 10.1093/carcin/bgp059. Epub 2009 Mar 6. Carcinogenesis. 2009. PMID: 19269999 Free PMC article.
-
3-O-methylfunicone, a selective inhibitor of mammalian Y-family DNA polymerases from an Australian sea salt fungal strain.Mar Drugs. 2009 Nov 23;7(4):624-39. doi: 10.3390/md7040624. Mar Drugs. 2009. PMID: 20098603 Free PMC article.
-
β-Sitosteryl (6'-O-linoleoyl)-glucoside of soybean (Glycine max L.) crude extract inhibits Y-family DNA polymerases.J Oleo Sci. 2010;59(11):621-30. doi: 10.5650/jos.59.621. J Oleo Sci. 2010. PMID: 20972363
-
Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose.Pharm Res. 2009 Sep;26(9):2066-80. doi: 10.1007/s11095-009-9932-0. Epub 2009 Jul 2. Pharm Res. 2009. PMID: 19575286 Free PMC article. Review.
Cited by
-
Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy.Viruses. 2018 Apr 17;10(4):201. doi: 10.3390/v10040201. Viruses. 2018. PMID: 29673174 Free PMC article.
-
Comprehensive analysis of telomerase inhibition by gallotannin.Oncotarget. 2018 Apr 10;9(27):18712-18719. doi: 10.18632/oncotarget.24642. eCollection 2018 Apr 10. Oncotarget. 2018. PMID: 29721155 Free PMC article.
-
Mangifera indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action.Breast Cancer (Dove Med Press). 2021 Sep 13;13:471-503. doi: 10.2147/BCTT.S316667. eCollection 2021. Breast Cancer (Dove Med Press). 2021. PMID: 34548817 Free PMC article. Review.
-
Biological and biomedical functions of Penta-O-galloyl-D-glucose and its derivatives.J Nat Med. 2014 Jul;68(3):465-72. doi: 10.1007/s11418-014-0823-2. Epub 2014 Feb 15. J Nat Med. 2014. PMID: 24532420 Review.
-
Pentagalloylglucose Inhibits the Replication of Rabies Virus via Mediation of the miR-455/SOCS3/STAT3/IL-6 Pathway.J Virol. 2019 Aug 28;93(18):e00539-19. doi: 10.1128/JVI.00539-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243136 Free PMC article.
References
-
- DePamphilis M. DNA replication in eukaryotic cells. Cold Spring Harbor Laboratory Press; 1996.
-
- Kornberg K, Baker TA. DNA replication. New York: Freeman W. D. and Co; 1992.
-
- Hubscher U, Maga G, Spadari S. Eukaryotic DNA polymerases. Annual review of biochemistry. 2002;71:133–63. - PubMed
-
- Bebenek K, Kunkel TA. Functions of DNA polymerases. Advances in protein chemistry. 2004;69:137–65. - PubMed
-
- Takata K, Shimizu T, Iwai S, Wood RD. Human DNA polymerase N (POLN) is a low fidelity enzyme capable of error-free bypass of 5S-thymine glycol. The Journal of biological chemistry. 2006;281:23445–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

