Post-ischemic conditioning in the rat retina is dependent upon ischemia duration and is not additive with ischemic pre-conditioning
- PMID: 20599964
- PMCID: PMC2976837
- DOI: 10.1016/j.exer.2010.06.015
Post-ischemic conditioning in the rat retina is dependent upon ischemia duration and is not additive with ischemic pre-conditioning
Abstract
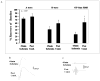
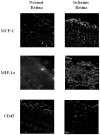
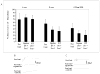
Ischemic pre-conditioning (IPC) provides neuroprotection in the rat retina from the damaging effects of severe ischemia. Recently, neuroprotection by retinal ischemic post-conditioning (Post-C), i.e., transient ischemia after more lengthy, damaging ischemia, was described, but its mechanisms are not yet known. One possible explanation of the effectiveness of Post-C is that it augments intrinsic neuroprotective mechanisms initiated during ischemia. Increasing duration of the damaging ischemic insult may therefore impact the effectiveness of Post-C. IPC, in contrast, sets in motion a series of neuroprotective events prior to the onset of ischemia. Thus, IPC and Post-C may operate by differing mechanisms. Accordingly, we examined the effect of retinal ischemic duration on post-ischemic outcome in vivo in rats after adding Post-C, and the impact of combining pre- and post-conditioning. Recovery after ischemia performed 24 h after IPC, or after Post-C performed 5 min after ischemia ended, was assessed functionally (electroretinography) and histologically at 7 days after ischemia. Durations of ischemia of 45 and 55 min were studied. Since recovery with IPC or Post-C alone, with 55 min of ischemia, did not achieve the same degree of effect (i.e., not complete recovery) exhibited in our previous studies of IPC using a different ischemia model, we also combined IPC and Post-C to test the hypothesis of the possible additive effects of the IPC and Post-C. We found that the recovery after Post-C was enhanced to a greater degree when ischemia was of longer duration. Post-C led to greater post-ischemic recovery compared to IPC. Both IPC and Post-C also attenuated structural damage to the retina. Contrary to our hypothesis, IPC and Post-C did not combine to enhance recovery after ischemia. In earlier studies, IPC attenuated post-ischemic apoptosis. To begin to examine the mechanism of Post-C, we studied its impact on apoptosis following ischemia. We examined apoptosis by determining the percentage of TUNEL-positive cells at 24 h after ischemia. Post-C attenuated apoptosis, but when combined with IPC, TUNEL was similar in the combined group to that of ischemia alone. We also examined the role of the recruitment of an inflammatory response in ischemia and Post-C. We found that inflammatory markers increased by ischemia were not altered by Post-C. We conclude that Post-C effectiveness depends upon the duration of ischemia; Post-C is not additive with IPC, and Post-C functions, in part, by preventing apoptotic damage to the inner retina. Post-C has considerable promise for clinical translation to eye diseases that cause blindness by ischemia.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
There is no proprietary interest for any of the authors.
Figures




References
-
- Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–213. - PubMed
-
- Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol. 2005;289:H1618–1626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

