Abeta(1-40) forms five distinct amyloid structures whose beta-sheet contents and fibril stabilities are correlated
- PMID: 20600131
- PMCID: PMC2919579
- DOI: 10.1016/j.jmb.2010.06.023
Abeta(1-40) forms five distinct amyloid structures whose beta-sheet contents and fibril stabilities are correlated
Abstract
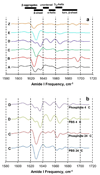
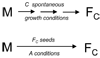
The ability of a single polypeptide sequence to grow into multiple stable amyloid fibrils sets these aggregates apart from most native globular proteins. The existence of multiple amyloid forms is the basis for strain effects in yeast prion biology, and might contribute to variations in Alzheimer's disease pathology. However, the structural basis for amyloid polymorphism is poorly understood. We report here five structurally distinct fibrillar aggregates of the Alzheimer's plaque peptide Abeta(1-40), as well as a non-fibrillar aggregate induced by Zn(2+). Each of these conformational forms exhibits a unique profile of physical properties, and all the fibrillar forms breed true in elongation reactions under a common set of growth conditions. Consistent with their defining cross-beta structure, we find that in this series the amyloid fibrils containing more extensive beta-sheet exhibit greater stability. At the same time, side chain packing outside of the beta-sheet regions contributes to stability, and to differences of stability between polymorphic forms. Stability comparison is facilitated by the unique feature that the free energy of the monomer (equivalent to the unfolded state in a protein folding reaction) does not vary, and hence can be ignored, in the comparison of DeltaG degrees of elongation values for each polymorphic fibril obtained under a single set of conditions.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures







References
-
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. [published erratum appears in N Engl J Med 1999 Oct 28;341(18):1407] - PubMed
-
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. - PubMed
-
- Serpell LC, Fraser PE, Sunde M. X-ray fiber diffraction of amyloid fibrils. Meth Enzymol. 1999;309:526–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

