WRN participates in translesion synthesis pathway through interaction with NBS1
- PMID: 20600238
- PMCID: PMC2911442
- DOI: 10.1016/j.mad.2010.06.005
WRN participates in translesion synthesis pathway through interaction with NBS1
Abstract
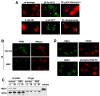
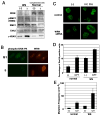
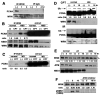
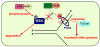
Werner syndrome (WS), caused by mutation of the WRN gene, is an autosomal recessive disorder associated with premature aging and predisposition to cancer. WRN belongs to the RecQ DNA helicase family, members of which play a role in maintaining genomic stability. Here, we demonstrate that WRN rapidly forms discrete nuclear foci in an NBS1-dependent manner following DNA damage. NBS1 physically interacts with WRN through its FHA domain, which interaction is important for the phosphorylation of WRN. WRN subsequently forms DNA damage-dependent foci during the S phase, but not in the G1 phase. WS cells exhibit an increase in spontaneous focus formation of poleta and Rad18, which are important for translesion synthesis (TLS). WRN also interacts with PCNA in the absence of DNA damage, but DNA damage induces the dissociation of PCNA from WRN, leading to the ubiquitination of PCNA, which is essential for TLS. This dissociation correlates with ATM/NBS1-dependent degradation of WRN. Moreover, WS cells show constitutive ubiquitination of PCNA and interaction between PCNA and Rad18 E3 ligase in the absence of DNA damage. Taken together, these results indicate that WRN participates in the TLS pathway to prevent genomic instability in an ATM/NBS1-dependent manner.
(c) 2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

Similar articles
-
WRN is required for ATM activation and the S-phase checkpoint in response to interstrand cross-link-induced DNA double-strand breaks.Mol Biol Cell. 2008 Sep;19(9):3923-33. doi: 10.1091/mbc.e07-07-0698. Epub 2008 Jul 2. Mol Biol Cell. 2008. PMID: 18596239 Free PMC article.
-
Werner's syndrome protein is phosphorylated in an ATR/ATM-dependent manner following replication arrest and DNA damage induced during the S phase of the cell cycle.Oncogene. 2003 Mar 13;22(10):1491-500. doi: 10.1038/sj.onc.1206169. Oncogene. 2003. PMID: 12629512
-
The Werner syndrome protein: linking the replication checkpoint response to genome stability.Aging (Albany NY). 2011 Mar;3(3):311-8. doi: 10.18632/aging.100293. Aging (Albany NY). 2011. PMID: 21389352 Free PMC article. Review.
-
ATR and ATM differently regulate WRN to prevent DSBs at stalled replication forks and promote replication fork recovery.EMBO J. 2010 Sep 15;29(18):3156-69. doi: 10.1038/emboj.2010.205. Epub 2010 Aug 27. EMBO J. 2010. PMID: 20802463 Free PMC article.
-
Werner syndrome protein: functions in the response to DNA damage and replication stress in S-phase.Exp Gerontol. 2007 Sep;42(9):871-8. doi: 10.1016/j.exger.2007.04.011. Epub 2007 May 10. Exp Gerontol. 2007. PMID: 17587522 Review.
Cited by
-
Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair.Prog Biophys Mol Biol. 2015 Mar;117(2-3):182-193. doi: 10.1016/j.pbiomolbio.2014.12.004. Epub 2015 Jan 7. Prog Biophys Mol Biol. 2015. PMID: 25576492 Free PMC article. Review.
-
Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks.DNA Repair (Amst). 2010 Dec 10;9(12):1299-306. doi: 10.1016/j.dnarep.2010.10.001. Epub 2010 Oct 28. DNA Repair (Amst). 2010. PMID: 21035407 Free PMC article. Review.
-
Nucleolar protein nucleolin functions in replication stress-induced DNA damage responses.J Radiat Res. 2019 May 1;60(3):281-288. doi: 10.1093/jrr/rry114. J Radiat Res. 2019. PMID: 30839063 Free PMC article.
-
Targeting an Achilles' heel of cancer with a WRN helicase inhibitor.Cell Cycle. 2013 Oct 15;12(20):3329-35. doi: 10.4161/cc.26320. Epub 2013 Sep 12. Cell Cycle. 2013. PMID: 24036544 Free PMC article.
-
RECQ helicases are deregulated in hematological malignancies in association with a prognostic value.Biomark Res. 2016 Feb 13;4:3. doi: 10.1186/s40364-016-0057-4. eCollection 2016. Biomark Res. 2016. PMID: 26877874 Free PMC article.
References
-
- Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
-
- Campbell MR, Wang Y, Andrew SE, Liu Y. Msh2 deficiency leads to chromosomal abnormalities, centrosome amplification, and telomere capping defect. Oncogene. 2006;25:2531–2536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

