Galectin-3: A novel substrate for c-Abl kinase
- PMID: 20600357
- PMCID: PMC2923841
- DOI: 10.1016/j.bbamcr.2010.06.007
Galectin-3: A novel substrate for c-Abl kinase
Abstract
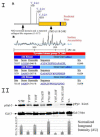
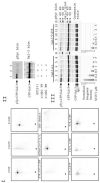
Galectin-3, a beta-galactoside-binding lectin, is found in cellular and extracellular location of the cell and has pleiotropic biological functions such as cell growth, cell adhesion and cell-cell interaction. It may exhibit anti- or pro-apoptotic activity depending on its localization and post-translational modifications. Two important post-translational modifications of galectin-3 have been reported: its cleavage and phosphorylation. Cleavage of galectin-3 was reported to be involved with angiogenic potential and apoptotic resistance. Phosphorylation of galectin-3 regulates its sugar-binding ability. In this report we have identified novel tyrosine phosphorylation sites in galectin-3 as well as the kinase responsible for its phosphorylation. Our results demonstrate that tyrosines at positions 79, 107 and 118 can be phosphorylated in vitro and in vivo by c-Abl kinase. Tyrosine 107 is the main target of c-Abl. Expression of galectin-3 Y107F mutant in galectin-3 null SK-Br-3 cells leads to morphological changes and increased motility compared to wild type galectin-3. Further investigation is needed to better understand the functional significance of the novel tyrosine phosphorylated sites of galectin-3.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage.J Biol Chem. 2012 Feb 17;287(8):5192-8. doi: 10.1074/jbc.C111.331686. Epub 2012 Jan 9. J Biol Chem. 2012. PMID: 22232548 Free PMC article.
-
Cleavage and phosphorylation: important post-translational modifications of galectin-3.Cancer Metastasis Rev. 2017 Jun;36(2):367-374. doi: 10.1007/s10555-017-9666-0. Cancer Metastasis Rev. 2017. PMID: 28378189 Review.
-
Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest.J Biol Chem. 2002 Mar 1;277(9):6852-7. doi: 10.1074/jbc.M107668200. Epub 2001 Nov 27. J Biol Chem. 2002. PMID: 11724777
-
c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3.Cell Death Differ. 2010 Aug;17(8):1277-87. doi: 10.1038/cdd.2010.8. Epub 2010 Feb 12. Cell Death Differ. 2010. PMID: 20150913
-
From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility.Curr Opin Neurobiol. 2000 Feb;10(1):80-7. doi: 10.1016/s0959-4388(99)00058-6. Curr Opin Neurobiol. 2000. PMID: 10679439 Review.
Cited by
-
AKAP12 deficiency impairs VEGF-induced endothelial cell migration and sprouting.Acta Physiol (Oxf). 2020 Jan;228(1):e13325. doi: 10.1111/apha.13325. Epub 2019 Jun 21. Acta Physiol (Oxf). 2020. PMID: 31162891 Free PMC article.
-
Eosinophil-expressed galectin-3 regulates cell trafficking and migration.Front Pharmacol. 2013 Apr 5;4:37. doi: 10.3389/fphar.2013.00037. eCollection 2013. Front Pharmacol. 2013. PMID: 23576987 Free PMC article.
-
Galectin-1 and Galectin-3 in B-Cell Precursor Acute Lymphoblastic Leukemia.Int J Mol Sci. 2022 Nov 18;23(22):14359. doi: 10.3390/ijms232214359. Int J Mol Sci. 2022. PMID: 36430839 Free PMC article.
-
Phosphorylation events during viral infections provide potential therapeutic targets.Rev Med Virol. 2012 May;22(3):166-81. doi: 10.1002/rmv.722. Epub 2011 Nov 24. Rev Med Virol. 2012. PMID: 22113983 Free PMC article.
-
Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17468-73. doi: 10.1073/pnas.1111138108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987825 Free PMC article.
References
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. - PubMed
-
- Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer. Int. J. Oncol. 2004;25:983–992. - PubMed
-
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim. Biophys. Acta. 2006;1760:616–635. - PubMed
-
- Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J. Biol. Chem. 1993;268:26712–26718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

