Vectored vaccines to protect against PRRSV
- PMID: 20600388
- PMCID: PMC7114413
- DOI: 10.1016/j.virusres.2010.06.017
Vectored vaccines to protect against PRRSV
Abstract
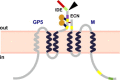
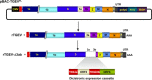
PRRSV is the causative agent of the most important infectious disease affecting swine herds worldwide, producing great economic losses. Commercially available vaccines are only partially effective in protection against PRRSV. Moreover, modified live vaccines may allow virus shedding, and could revert generating virulent phenotypes. Therefore, new efficient vaccines are required. Vaccines based on recombinant virus genomes (virus vectored vaccines) against PRRSV could represent a safe alternative for the generation of modified live vaccines. In this paper, current vectored vaccines to protect against PRRSV are revised, including those based on pseudorabies virus, poxvirus, adenovirus, and virus replicons. Special attention has been provided to the use of transmissible gastroenteritis virus (TGEV) as vector for the expression of PRRSV antigens. This vector has the capability of expressing high levels of heterologous genes, is a potent interferon-α inducer, and presents antigens in mucosal surfaces, eliciting both secretory and systemic immunity. A TGEV derived vector (rTGEV) was generated, expressing PRRSV wild type or modified GP5 and M proteins, described as the main inducers of neutralizing antibodies and cellular immune response, respectively. Protection experiments showed that vaccinated animals developed a faster and stronger humoral immune response than the non-vaccinated ones. Partial protection in challenged animals was observed, as vaccinated pigs showed decreased lung damage when compared with the non-vaccinated ones. Nevertheless, the level of neutralizing antibodies was low, what may explain the limited protection observed. Several strategies are proposed to improve current rTGEV vectors expressing PRRSV antigens.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998;18:485–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

