Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels
- PMID: 20600597
- PMCID: PMC3365921
- DOI: 10.1016/j.neulet.2010.06.064
Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels
Abstract
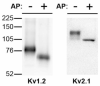
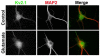
Phosphorylation is the most common and abundant post-translational modification to eukaryotic proteins, regulating a plethora of dynamic cellular processes. Here, we review and discuss recent advances in our knowledge of the breadth and importance of reversible phosphorylation in regulating the expression, localization and function of mammalian neuronal voltage-gated potassium (Kv) channels, key regulators of neuronal function. We highlight the role of modern mass spectrometric techniques and phosphospecific antibodies in revealing the extent and nature of phosphorylation at specific sites in Kv channels. We also emphasize the role of reversible phosphorylation in dynamically regulating diverse aspects of Kv channel biology. Finally, we discuss as important future directions the determination of the mechanistic basis for how altering phosphorylation state affects Kv channel expression, localization and function, the nature of macromolecular signaling complexes containing Kv channels and enzymes regulating their phosphorylation state, and the specific role of Kv channel phosphorylation in regulating neuronal function during physiological and pathophysiological events.
Copyright © 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Diverse roles for auxiliary subunits in phosphorylation-dependent regulation of mammalian brain voltage-gated potassium channels.Pflugers Arch. 2011 Nov;462(5):631-43. doi: 10.1007/s00424-011-1004-8. Epub 2011 Aug 6. Pflugers Arch. 2011. PMID: 21822597 Free PMC article. Review.
-
Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration.Transl Stroke Res. 2014 Feb;5(1):38-58. doi: 10.1007/s12975-013-0297-7. Epub 2013 Nov 19. Transl Stroke Res. 2014. PMID: 24323720 Free PMC article. Review.
-
Determinants of voltage-gated potassium channel surface expression and localization in Mammalian neurons.Crit Rev Biochem Mol Biol. 2004 May-Jun;39(3):125-45. doi: 10.1080/10409230490475417. Crit Rev Biochem Mol Biol. 2004. PMID: 15596548 Review.
-
Neuronal voltage-gated K+ (Kv) channels function in macromolecular complexes.Neurosci Lett. 2010 Dec 10;486(2):73-7. doi: 10.1016/j.neulet.2010.08.067. Epub 2010 Sep 9. Neurosci Lett. 2010. PMID: 20813163 Free PMC article. Review.
-
Differential effect of brief electrical stimulation on voltage-gated potassium channels.J Neurophysiol. 2017 May 1;117(5):2014-2024. doi: 10.1152/jn.00915.2016. Epub 2017 Feb 15. J Neurophysiol. 2017. PMID: 28202576 Free PMC article.
Cited by
-
Diverse roles for auxiliary subunits in phosphorylation-dependent regulation of mammalian brain voltage-gated potassium channels.Pflugers Arch. 2011 Nov;462(5):631-43. doi: 10.1007/s00424-011-1004-8. Epub 2011 Aug 6. Pflugers Arch. 2011. PMID: 21822597 Free PMC article. Review.
-
Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries.Circ Res. 2014 Apr 11;114(8):1258-67. doi: 10.1161/CIRCRESAHA.114.303167. Epub 2014 Feb 28. Circ Res. 2014. PMID: 24585759 Free PMC article.
-
Molecular determinants of beta-adrenergic signaling to voltage-gated K+ channels in the cerebral circulation.Microcirculation. 2018 Jan;25(1):10.1111/micc.12425. doi: 10.1111/micc.12425. Microcirculation. 2018. PMID: 29072364 Free PMC article. Review.
-
Increased KV2.1 Channel Clustering Underlies the Reduction of Delayed Rectifier K+ Currents in Hippocampal Neurons of the Tg2576 Alzheimer's Disease Mouse.Cells. 2022 Sep 9;11(18):2820. doi: 10.3390/cells11182820. Cells. 2022. PMID: 36139395 Free PMC article.
-
Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus.J Neurosci. 2014 Apr 16;34(16):5486-96. doi: 10.1523/JNEUROSCI.4861-12.2014. J Neurosci. 2014. PMID: 24741039 Free PMC article.
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275:5337–5346. - PubMed
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Cohen P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci. 2000;25:596–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

