Comparison of metabolic and neuropathy profiles of rats with streptozotocin-induced overt and moderate insulinopenia
- PMID: 20600635
- PMCID: PMC2926213
- DOI: 10.1016/j.neuroscience.2010.06.059
Comparison of metabolic and neuropathy profiles of rats with streptozotocin-induced overt and moderate insulinopenia
Abstract
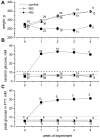
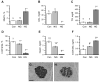
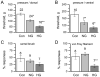
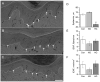
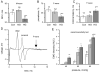
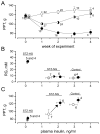
To assess the relative roles of insulinopenia, hyperglycemia and dyslipidemia in pathogenesis of diabetic neuropathy, we compared plasma insulin, glucose and lipid metabolism and peripheral nerve function in rats with streptozotocin (STZ)-induced overt and moderate insulinopenia (hyperglycemic, STZ-HG; random glucose>11 mM and normoglycemic, STZ-NG rats). While being slightly insulinopenic, STZ-NG rats are metabolically not different from control, naive animals, by having normal glucose tolerance and normal levels of plasma glucose, glycated HbA1c, cholesterol and triglycerides. Two weeks following injection of STZ, STZ-HG but not STZ-NG rats had suppressed motor nerve conduction velocity, F-wave prevalence, withdrawal responses to heat and von Frey filament stimuli. In apparent correlation with plasma insulin level, both STZ-HG and -NG rats manifested exaggerated responses in paw pressure and colorectal distension tests. These data suggest that insulinopenia may play a leading role in the diabetic impairment of deep muscle and visceral afferent pathways while hyperglycemia/dyslipidemia may represent a key requirement for the onset and progression of electrophysiological nerve impairment and loss of superficial heat and tactile perception. STZ-NG rats offer a convenient model for the investigation of the short-term effects of insulinopenia on peripheral nerve function.
Copyright 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
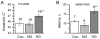
References
-
- Bove G. Mechanical sensory threshold testing using nylon monofilaments: the pain field's “tin standard”. Pain. 2006;124:13–17. - PubMed
-
- Bravi MC, Armiento A, Laurenti O, Cassone-Faldetta M, De Luca O, Moretti A, De Mattia G. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2006;55:691–695. - PubMed
-
- Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830. - PubMed
-
- Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

