Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation
- PMID: 20600644
- PMCID: PMC2918713
- DOI: 10.1016/j.neuroscience.2010.06.047
Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation
Abstract
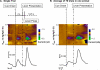
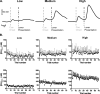
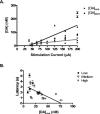
The mesolimbic dopamine system is critically involved in modulating reward-seeking behavior and is transiently activated upon presentation of reward-predictive cues. It has previously been shown, using fast-scan cyclic voltammetry in behaving rats, that cues predicting a variety of reinforcers including food/water, cocaine or intracranial self-stimulation (ICSS) elicit time-locked transient fluctuations in dopamine concentration in the nucleus accumbens (NAc) shell. These dopamine transients have been found to correlate with reward-related learning and are believed to promote reward-seeking behavior. Here, we investigated the effects of varying reinforcer magnitude (intracranial stimulation parameters) on cue-evoked dopamine release in the NAc shell in rats performing ICSS. We found that the amplitude of cue-evoked dopamine is adaptable, tracks reinforcer magnitude and is significantly correlated with ICSS seeking behavior. Specifically, the concentration of cue-associated dopamine transients increased significantly with increasing reinforcer magnitude, while, at the same time, the latency to lever press decreased with reinforcer magnitude. These data support the proposed role of NAc dopamine in the facilitation of reward-seeking and provide unique insight into factors influencing the plasticity of dopaminergic signaling during behavior.
(c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
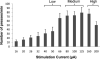
References
-
- Arvanitogiannis A, Shizgal P. The reinforcement mountain: allocation of behavior as a function of the rate and intensity of rewarding brain stimulation. Behav Neurosci. 2008;122:1126–1138. - PubMed
-
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. - PubMed
-
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 1980;185:1–15. - PubMed
-
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

