Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma
- PMID: 20600672
- PMCID: PMC2926211
- DOI: 10.1016/j.neuroscience.2010.06.017
Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma
Abstract
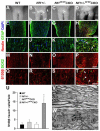
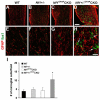
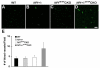
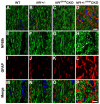
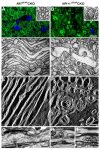
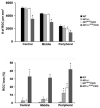
The purpose of this study was to investigate the progression of changes in retinal ganglion cells and optic nerve glia in neurofibromatosis-1 (NF1) genetically-engineered mice with optic glioma. Optic glioma tumors were generated in Nf1+/- mice lacking Nf1 expression in GFAP+ cells (astrocytes). Standard immunohistochemistry methods were employed to identify astrocytes (GFAP, S100beta), proliferating progenitor cells (sox2, nestin), microglia (Iba1), endothelial cells (CD31) and retinal ganglion cell (RGC) axons (Neurofilament 68k) in Nf1+/-, Nf1(GFAP)CKO (wild-type mice with Nf1 loss in glial cells), and Nf1+/-(GFAP)CKO (Nf1+/- mice with Nf1 loss in glial cells) mice. Ultrastructural changes in the optic chiasm and nerve were assessed by electron microscopy (EM). RGC were counted in whole retina preparations using high-resolution, mosaic confocal microscopy following their delineation by retrograde FluoroGold labeling. We found that only Nf1+/-(GFAP)CKO mice exhibited gross pre-chiasmatic optic nerve and chiasm enlargements containing aggregated GFAP+/nestin+ and S100beta+/sox2+ cells (neoplastic glia) as well as increased numbers of blood vessels and microglia. Optic gliomas in Nf1+/-(GFAP)CKO mice contained axon fiber irregularities and multilamellar bodies of degenerated myelin. EM and EM tomographic analyses showed increased glial disorganization, disoriented axonal projections, profiles of degenerating myelin and structural alterations at nodes of Ranvier. Lastly, we found reduced RGC numbers in Nf1+/-(GFAP)CKO mice, supporting a model in which the combination of optic nerve Nf1 heterozygosity and glial cell Nf1 loss results in disrupted axonal-glial relationships, subsequently culminating in the degeneration of optic nerve axons and loss of their parent RGC neurons.
Copyright 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1.J Neurosci Res. 2019 Jan;97(1):45-56. doi: 10.1002/jnr.24250. Epub 2018 Apr 28. J Neurosci Res. 2019. PMID: 29704429 Free PMC article. Review.
-
Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma.J Neuropathol Exp Neurol. 2009 May;68(5):542-51. doi: 10.1097/NEN.0b013e3181a3240b. J Neuropathol Exp Neurol. 2009. PMID: 19525901 Free PMC article.
-
The impact of coexisting genetic mutations on murine optic glioma biology.Neuro Oncol. 2015 May;17(5):670-7. doi: 10.1093/neuonc/nou287. Epub 2014 Sep 21. Neuro Oncol. 2015. PMID: 25246427 Free PMC article.
-
NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1.Hum Mol Genet. 2016 May 1;25(9):1703-13. doi: 10.1093/hmg/ddw039. Epub 2016 Feb 16. Hum Mol Genet. 2016. PMID: 26908603 Free PMC article.
-
Human stem cell modeling in neurofibromatosis type 1 (NF1).Exp Neurol. 2018 Jan;299(Pt B):270-280. doi: 10.1016/j.expneurol.2017.04.001. Epub 2017 Apr 6. Exp Neurol. 2018. PMID: 28392281 Free PMC article. Review.
Cited by
-
Challenges in Drug Discovery for Neurofibromatosis Type 1-Associated Low-Grade Glioma.Front Oncol. 2016 Dec 20;6:259. doi: 10.3389/fonc.2016.00259. eCollection 2016. Front Oncol. 2016. PMID: 28066715 Free PMC article. Review.
-
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1.J Neurosci Res. 2019 Jan;97(1):45-56. doi: 10.1002/jnr.24250. Epub 2018 Apr 28. J Neurosci Res. 2019. PMID: 29704429 Free PMC article. Review.
-
RNA Sequencing of Tumor-Associated Microglia Reveals Ccl5 as a Stromal Chemokine Critical for Neurofibromatosis-1 Glioma Growth.Neoplasia. 2015 Oct;17(10):776-88. doi: 10.1016/j.neo.2015.10.002. Neoplasia. 2015. PMID: 26585233 Free PMC article.
-
Brain tumors in Neurofibromatosis type 1.Neurooncol Adv. 2019 Oct 26;1(1):vdz040. doi: 10.1093/noajnl/vdz040. eCollection 2019 May-Dec. Neurooncol Adv. 2019. PMID: 32642668 Free PMC article. Review.
-
Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations.Ann Neurol. 2007 Mar;61(3):189-98. doi: 10.1002/ana.21107. Ann Neurol. 2007. PMID: 17387725 Free PMC article. Review.
References
-
- Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147–158. - PubMed
-
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
-
- Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54:106–113. - PubMed
-
- Bajenaru ML, Garbow JR, Perry A, Hernandez MR, Gutmann DH. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57:119–127. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

