Advances in developing HIV-1 viral load assays for resource-limited settings
- PMID: 20600784
- PMCID: PMC2946488
- DOI: 10.1016/j.biotechadv.2010.06.004
Advances in developing HIV-1 viral load assays for resource-limited settings
Abstract
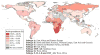
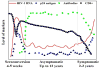
Commercial HIV-1 RNA viral load assays have been routinely used in developed countries to monitor antiretroviral treatment (ART). However, these assays require expensive equipment and reagents, well-trained operators, and established laboratory infrastructure. These requirements restrict their use in resource-limited settings where people are most afflicted with the HIV-1 epidemic. Inexpensive alternatives such as the Ultrasensitive p24 assay, the reverse transcriptase (RT) assay and in-house reverse transcription quantitative polymerase chain reaction (RT-qPCR) have been developed. However, they are still time-consuming, technologically complex and inappropriate for decentralized laboratories as point-of-care (POC) tests. Recent advances in microfluidics and nanotechnology offer new strategies to develop low-cost, rapid, robust and simple HIV-1 viral load monitoring systems. We review state-of-the-art technologies used for HIV-1 viral load monitoring in both developed and developing settings. Emerging approaches based on microfluidics and nanotechnology, which have potential to be integrated into POC HIV-1 viral load assays, are also discussed.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Abbott. Abbott RealTime HIV-1 (package insert) 2007.
-
- Bhattacharyya A, Klapperich CM. Design and testing of a disposable microfluidic chemiluminescent immunoassay for disease biomarkers in human serum samples. Biomed Microdevices. 2007;9:245–51. - PubMed
-
- Bonard D, Rouet F, Toni TA, Minga A, Huet C, Ekouevi DK, et al. Field evaluation of an improved assay using a heat-dissociated p24 antigen for adults mainly infected with HIV-1 CRF02_AG strains in Cote d’Ivoire, West Africa. J Acquir Immune Defic Syndr. 2003;34:267–73. - PubMed
-
- Boni J, Opravil M, Tomasik Z, Rothen M, Bisset L, Grob PJ, et al. Simple monitoring of antiretroviral therapy with a signal-amplification-boosted HIV-1 p24 antigen assay with heat-denatured plasma. Aids. 1997;11:F47–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

