Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency
- PMID: 20600873
- PMCID: PMC3117462
- DOI: 10.1016/j.bbadis.2010.06.007
Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency
Abstract
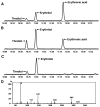
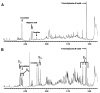
Background: Sedoheptulose, arabitol, ribitol, and erythritol have been identified as key diagnostic metabolites in TALDO deficiency.
Method: Urine from 6 TALDO-deficient patients and TALDO-deficient knock-out mice were analyzed using ¹H-NMR spectroscopy and GC-mass spectrometry.
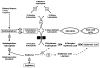
Results: Our data confirm the known metabolic characteristics in TALDO-deficient patients. The β-furanose form was the major sedoheptulose anomer in TALDO-deficient patients. Erythronic acid was identified as a major abnormal metabolite in all patients and in knock-out TALDO mice implicating an as yet unknown biochemical pathway in this disease. A putative sequence of enzymatic reactions leading to the formation of erythronic acid is presented. The urinary concentration of the citric acid cycle intermediates 2-oxoglutaric acid and fumaric acid was increased in the majority of TALDO-deficient patients but not in the knock-out mice.
Conclusion: Erythronic acid is a novel and major hallmark in TALDO deficiency. The pathway leading to its production may play a role in healthy humans as well. In TALDO-deficient patients, there is an increased flux through this pathway. The finding of increased citric acid cycle intermediates hints toward a disturbed mitochondrial metabolism in TALDO deficiency.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


References
-
- Verhoeven NM, Wallot M, Huck JH, Dirsch O, Ballauf A, Neudorf U, Salomons GS, van der Knaap MS, Voit T, Jakobs C. A newborn with severe liver failure, cardiomyopathy and transaldolase deficiency. J Inherit Metab Dis. 2005;28:169–179. - PubMed
-
- Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008;31:703–717. - PubMed
-
- Tylki-Szymanska A, Stradomska TJ, Wamelink MM, Salomons GS, Taybert J, Pawlowska J, Jakobs C. Transaldolase deficiency in two new patients with a relative mild phenotype. Mol Genet Metab. 2009;97:15–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

