All y'all need to know 'bout retroelements in cancer
- PMID: 20600922
- PMCID: PMC2943028
- DOI: 10.1016/j.semcancer.2010.06.001
All y'all need to know 'bout retroelements in cancer
Abstract
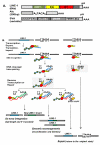
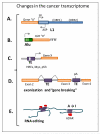
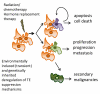
Genetic instability is one of the principal hallmarks and causative factors in cancer. Human transposable elements (TE) have been reported to cause human diseases, including several types of cancer through insertional mutagenesis of genes critical for preventing or driving malignant transformation. In addition to retrotransposition-associated mutagenesis, TEs have been found to contribute even more genomic rearrangements through non-allelic homologous recombination. TEs also have the potential to generate a wide range of mutations derivation of which is difficult to directly trace to mobile elements, including double strand breaks that may trigger mutagenic genomic rearrangements. Genome-wide hypomethylation of TE promoters and significantly elevated TE expression in almost all human cancers often accompanied by the loss of critical DNA sensing and repair pathways suggests that the negative impact of mobile elements on genome stability should increase as human tumors evolve. The biological consequences of elevated retroelement expression, such as the rate of their amplification, in human cancers remain obscure, particularly, how this increase translates into disease-relevant mutations. This review is focused on the cellular mechanisms that control human TE-associated mutagenesis in cancer and summarizes the current understanding of TE contribution to genetic instability in human malignancies.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–1037. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. - PubMed
-
- Paris PL, et al. Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum.Mol.Genet. 2004;13:1303–1313. - PubMed
-
- Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

