Mechanisms and modification of chlorine-induced lung injury in animals
- PMID: 20601632
- PMCID: PMC3136964
- DOI: 10.1513/pats.201001-009SM
Mechanisms and modification of chlorine-induced lung injury in animals
Abstract
Chlorine (Cl(2)) is a reactive oxidant gas used extensively in industrial processes. Exposure of both humans and animals to high concentrations of Cl(2) results in acute lung injury, which may resolve spontaneously or progress to acute respiratory failure. Injury to airway and alveolar epithelium may result from chemical reactions of Cl(2), from HOCl (the hydrolysis product of Cl(2)), and/or from the various reaction products, such as chloramines, that are formed from the reactions of these chlorinating species with biological molecules. Subsequent reactions may initiate self-propagating reactions and induce the production of inflammatory mediators compounding injury to pulmonary surfactant, ion channels, and components of lung epithelial and airway cells. Low-molecular-weight antioxidants, such as ascorbate, glutathione, and urate, present in the lung epithelial lining fluid and tissue, remove Cl(2) and HOCl and thus decrease injury to critical target biological targets. However, levels of lung antioxidants of animals exposed to Cl(2) in concentrations likely to be encountered in the vicinity of industrial accidents decrease rapidly and irreversibly. Our measurements show that prophylactic administration of a mixture containing ascorbate and desferal N-acetyl-cysteine, a precursor of reduced glutathione, prevents Cl(2)-induced injury to the alveolar epithelium of rats exposed to Cl(2). The clinical challenge is to deliver sufficient quantities of antioxidants noninvasively, after Cl(2) exposure, to decrease morbidity and mortality.
Figures

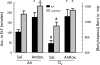
References
-
- Evans RB. Chlorine: state of the art. Lung 2005;183:151–167. - PubMed
-
- Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol 1998;36:87–93. - PubMed
-
- Cave D, Fadam A. Iraq insurgents employ chlorine in bomb attacks. New York Times, February 22, 2007.
-
- Sander R. Compilation of Henry's law constants for inorganic and organic species of potential importance in environmental chemistry. [Accessed May 2010.] Available from: http://www.mpch-mainz.mpg.de/∼sander/res/henry.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
