Multicolor single-molecule FRET to explore protein folding and binding
- PMID: 20601974
- PMCID: PMC3005188
- DOI: 10.1039/c003024d
Multicolor single-molecule FRET to explore protein folding and binding
Abstract
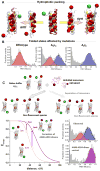
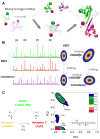
Proper protein function in cells, tissues and organisms depends critically on correct protein folding or interaction with partners. Over the last decade, single-molecule FRET (smFRET) has emerged as a powerful tool to probe complex distributions, dynamics, pathways and landscapes in protein folding and binding reactions, leveraging its ability to avoid averaging over an ensemble of molecules. While smFRET was practiced in a two-color form until recently, the last few years have seen the development of enhanced multicolor smFRET methods that provide additional structural information permitting us to probe more complex mechanisms. In this review, we provide a brief introduction to the smFRET technique, then follow with advanced multicolor measurements and end with ongoing methodology developments in microfluidics and protein labeling that are beginning to make these techniques more broadly applicable to answering a number of key questions about folding and binding.
Figures

Similar articles
-
A Multicolor Single-Molecule FRET Approach to Study Protein Dynamics and Interactions Simultaneously.Methods Enzymol. 2016;581:487-516. doi: 10.1016/bs.mie.2016.08.024. Epub 2016 Oct 10. Methods Enzymol. 2016. PMID: 27793290
-
Multicolor single-molecule FRET studies on dynamic protein systems.Curr Opin Struct Biol. 2025 Aug;93:103117. doi: 10.1016/j.sbi.2025.103117. Epub 2025 Jul 14. Curr Opin Struct Biol. 2025. PMID: 40664122 Review.
-
Single Molecule FRET Analysis of DNA Binding Proteins.Methods Mol Biol. 2018;1665:217-239. doi: 10.1007/978-1-4939-7271-5_12. Methods Mol Biol. 2018. PMID: 28940072
-
How the dyes affect folding of small proteins in single-molecule FRET experiments: A simulation study.Biophys Chem. 2019 Nov;254:106243. doi: 10.1016/j.bpc.2019.106243. Epub 2019 Aug 9. Biophys Chem. 2019. PMID: 31442765
-
Protein folding at single-molecule resolution.Biochim Biophys Acta. 2011 Aug;1814(8):1021-9. doi: 10.1016/j.bbapap.2011.01.011. Epub 2011 Feb 17. Biochim Biophys Acta. 2011. PMID: 21303706 Free PMC article. Review.
Cited by
-
Regulated unfolding of proteins in signaling.FEBS Lett. 2013 Apr 17;587(8):1081-8. doi: 10.1016/j.febslet.2013.02.024. Epub 2013 Feb 20. FEBS Lett. 2013. PMID: 23454209 Free PMC article. Review.
-
The statistics of protein expression ratios for cellular fluorescence studies.Eur Biophys J. 2012 Mar;41(3):341-52. doi: 10.1007/s00249-012-0792-x. Epub 2012 Feb 4. Eur Biophys J. 2012. PMID: 22307451 Free PMC article.
-
Transition Path Times Measured by Single-Molecule Spectroscopy.J Mol Biol. 2018 Feb 16;430(4):409-423. doi: 10.1016/j.jmb.2017.05.018. Epub 2017 May 25. J Mol Biol. 2018. PMID: 28551335 Free PMC article. Review.
-
Shedding light on protein folding landscapes by single-molecule fluorescence.Chem Soc Rev. 2014 Feb 21;43(4):1172-88. doi: 10.1039/c3cs60311c. Chem Soc Rev. 2014. PMID: 24336839 Free PMC article. Review.
-
Single-molecule fluorescence studies of intrinsically disordered proteins and liquid phase separation.Biochim Biophys Acta Proteins Proteom. 2019 Oct;1867(10):980-987. doi: 10.1016/j.bbapap.2019.04.007. Epub 2019 May 2. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31054969 Free PMC article. Review.
References
-
- Frauenfelder H, Sligar SG, Wolynes PG. Science. 1991;254:1598–1603. - PubMed
-
- Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Proteins-Structure Function and Genetics. 1995;21:167–195. - PubMed
-
- Onuchic JN, Luthey-Schulten Z, Wolynes PG. Annu Rev Phys Chem. 1997;48:545–600. - PubMed
-
- Dill KA, Chan HS. Nature Structural Biology. 1997;4:10–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

