Molecular aspects of cyclophilins mediating therapeutic actions of their ligands
- PMID: 20602248
- PMCID: PMC11115621
- DOI: 10.1007/s00018-010-0437-0
Molecular aspects of cyclophilins mediating therapeutic actions of their ligands
Abstract
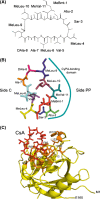
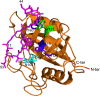
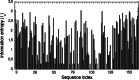
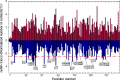
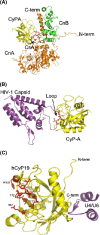
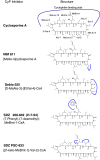
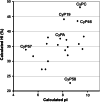
Cyclosporine A (CsA) is an immunosuppressive cyclic peptide that binds with a high affinity to 18 kDa human cyclophilin-A (hCyPA). CsA and its several natural derivatives have some pharmacological potential in treatment of diverse immune disorders. More than 20 paralogues of CyPA are expressed in the human body while expression levels and functions of numerous ORFs encoding cyclophilin-like sequences remain unknown. Certain derivatives of CsA devoid of immunosuppressive activity may have some potential in treatments of Alzheimer diseases, Hepatitis C and HIV infections, amyotrophic lateral sclerosis, congenital muscular dystrophy, asthma and various parasitic infections. Here, we discuss structural and functional aspects of the human cyclophilins and their interaction with various intra-cellular targets that can be under the control of CsA or its complexes with diverse cyclophilins that are selectively expressed in different cellular compartments. Some molecular aspects of the cyclophilins expressed in parasites invading humans and causing diseases were also analyzed.
Figures


Similar articles
-
From chemical tools to clinical medicines: nonimmunosuppressive cyclophilin inhibitors derived from the cyclosporin and sanglifehrin scaffolds.J Med Chem. 2014 Sep 11;57(17):7145-59. doi: 10.1021/jm500223x. Epub 2014 Jun 3. J Med Chem. 2014. PMID: 24831536
-
Structural Basis for Cyclosporin Isoform-Specific Inhibition of Cyclophilins from Toxoplasma gondii.ACS Infect Dis. 2023 Feb 10;9(2):365-377. doi: 10.1021/acsinfecdis.2c00566. Epub 2023 Jan 18. ACS Infect Dis. 2023. PMID: 36653744 Free PMC article.
-
Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication.Antiviral Res. 2020 Jan;173:104620. doi: 10.1016/j.antiviral.2019.104620. Epub 2019 Oct 18. Antiviral Res. 2020. PMID: 31634494 Free PMC article.
-
Cyclophilin inhibitors for the treatment of HCV infection.Curr Opin Investig Drugs. 2010 Aug;11(8):911-8. Curr Opin Investig Drugs. 2010. PMID: 20721833 Review.
-
Cyclophilin D as a drug target.Curr Med Chem. 2003 Aug;10(16):1485-506. doi: 10.2174/0929867033457160. Curr Med Chem. 2003. PMID: 12871122 Review.
Cited by
-
Trypanosoma cruzi Secreted Cyclophilin TcCyP19 as an Early Marker for Trypanocidal Treatment Efficiency.Int J Mol Sci. 2023 Jul 25;24(15):11875. doi: 10.3390/ijms241511875. Int J Mol Sci. 2023. PMID: 37569250 Free PMC article.
-
Cyclophilin A binds to the viral RNA and replication proteins, resulting in inhibition of tombusviral replicase assembly.J Virol. 2013 Dec;87(24):13330-42. doi: 10.1128/JVI.02101-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089553 Free PMC article.
-
Synthesis of the C21-C34 fragment of antascomicin B.Tetrahedron Lett. 2011 Oct 30;52(48):6349-6351. doi: 10.1016/j.tetlet.2011.09.027. Tetrahedron Lett. 2011. PMID: 22199407 Free PMC article.
-
Heterogeneous susceptibility of circulating SIV isolate capsids to HIV-interacting factors.Retrovirology. 2013 Jul 24;10:77. doi: 10.1186/1742-4690-10-77. Retrovirology. 2013. PMID: 23883001 Free PMC article.
-
Differential loss of prolyl isomerase or chaperone activity of Ran-binding protein 2 (Ranbp2) unveils distinct physiological roles of its cyclophilin domain in proteostasis.J Biol Chem. 2014 Feb 21;289(8):4600-25. doi: 10.1074/jbc.M113.538215. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403063 Free PMC article.
References
-
- Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporine A: a new antilymphocytic agent. Agents Actions. 1994;43:179–186. - PubMed
-
- Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporine A. Science. 1984;226:544–546. - PubMed
-
- Galat A, Riviere S. Peptidylprolyl cis/trans isomerases. Oxford: Oxford University Press; 1998.
-
- Edlich F, Fischer G. Pharmacological targeting of catalized protein folding: the example of peptide bond cis/trans isomerases. Hand Exp Pharmacol. 2006;172:359–404. - PubMed
-
- Galat A, Bouet F. Cyclophilin B is an abundant protein whose conformation is similar to that of cyclophilin A. FEBS Lett. 1994;347:31–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

