Validation of archived chemical shifts through atomic coordinates
- PMID: 20602353
- PMCID: PMC2970900
- DOI: 10.1002/prot.22756
Validation of archived chemical shifts through atomic coordinates
Abstract
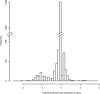
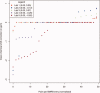
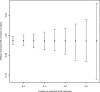
The public archives containing protein information in the form of NMR chemical shift data at the BioMagResBank (BMRB) and of 3D structure coordinates at the Protein Data Bank are continuously expanding. The quality of the data contained in these archives, however, varies. The main issue for chemical shift values is that they are determined relative to a reference frequency. When this reference frequency is set incorrectly, all related chemical shift values are systematically offset. Such wrongly referenced chemical shift values, as well as other problems such as chemical shift values that are assigned to the wrong atom, are not easily distinguished from correct values and effectively reduce the usefulness of the archive. We describe a new method to correct and validate protein chemical shift values in relation to their 3D structure coordinates. This method classifies atoms using two parameters: the per-atom solvent accessible surface area (as calculated from the coordinates) and the secondary structure of the parent amino acid. Through the use of Gaussian statistics based on a large database of 3220 BMRB entries, we obtain per-entry chemical shift corrections as well as Z scores for the individual chemical shift values. In addition, information on the error of the correction value itself is available, and the method can retain only dependable correction values. We provide an online resource with chemical shift, atom exposure, and secondary structure information for all relevant BMRB entries (http://www.ebi.ac.uk/pdbe/nmr/vasco) and hope this data will aid the development of new chemical shift-based methods in NMR.
2010 Wiley-Liss, Inc.
Figures

References
-
- Henrick K, Feng Z, Bluhm WF, Dimitropoulos D, Doreleijers JF, Dutta S, Flippen-Anderson JL, Ionides J, Kamada C, Krissinel E, Lawson CL, Markley JL, Nakamura H, Newman R, Shimizu Y, Swaminathan J, Velankar S, Ory J, Ulrich EL, Vranken W, Westbrook J, Yamashita R, Yang H, Young J, Yousufuddin M, Berman HM. Remediation of the protein data bank archive. Nucleic Acids Res. 2008;36:D426–D433. - PMC - PubMed
-
- Pardi A, Wagner G, Wüthrich K. Protein conformation and proton nuclear-magnetic-resonance chemical shifts. Eur J Biochem. 1983;137:445–454. - PubMed
-
- Wishart DS, Sykes BD, Richards FM. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J Mol Biol. 1991;222:311–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

