Pregabalin in fibromyalgia--responder analysis from individual patient data
- PMID: 20602781
- PMCID: PMC2906437
- DOI: 10.1186/1471-2474-11-150
Pregabalin in fibromyalgia--responder analysis from individual patient data
Abstract
Background: Population mean changes are difficult to use in clinical practice. Responder analysis may be better, but needs validating for level of response and treatment duration. A consensus group has defined what constitutes minimal, moderate, and substantial benefit based on pain intensity and Patient Global Impression of Change scores.
Methods: We obtained individual patient data from four randomised double blind trials of pregabalin in fibromyalgia lasting eight to 14 weeks. We calculated response for all efficacy outcomes using any improvement (>or= 0%), minimal improvement (>or= 15%), moderate improvement (>or= 30%), substantial improvement (>or= 50%), and extensive improvement (>or= 70%), with numbers needed to treat (NNT) for pregabalin 300 mg, 450 mg, and 600 mg daily compared with placebo.
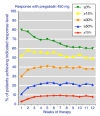
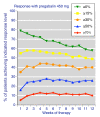
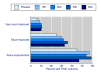
Results: Information from 2,757 patients was available. Pain intensity and sleep interference showed reductions with increasing level of response, a significant difference between pregabalin and placebo, and a trend towards lower (better) NNTs at higher doses. Maximum response rates occurred at 4-6 weeks for higher levels of response, and were constant thereafter. NNTs (with 95% confidence intervals) for >or= 50% improvement in pain intensity compared with placebo after 12 weeks were 22 (11 to 870) for pregabalin 300 mg, 16 (9.3 to 59) for pregabalin 450 mg, and 13 (8.1 to 31) for pregabalin 600 mg daily. NNTs for >or= 50% improvement in sleep interference compared with placebo after 12 weeks were 13 (8.2 to 30) for pregabalin 300 mg, 8.4 (6.0 to 14) for pregabalin 450 mg, and 8.4 (6.1 to 14) for pregabalin 600 mg. Other outcomes had fewer respondents at higher response levels, but generally did not discriminate between pregabalin and placebo, or show any dose response. Shorter duration and use of 'any improvement' over-estimated treatment effect compared with longer duration and higher levels of response.
Conclusions: Responder analysis is useful in fibromyalgia, particularly for pain and sleep outcomes. Some fibromyalgia patients treated with pregabalin experience a moderate or substantial pain response that is consistent over time. Short trials using 'any improvement' as an outcome overestimate treatment effects.
Figures
References
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. - DOI - PubMed
-
- Russell IJ, Raphael KG. Fibromyalgia syndrome: presentation, diagnosis, differential diagnosis, and vulnerability. CNS Spectr. 2008;13:6–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

