Ao38, a new cell line from eggs of the black witch moth, Ascalapha odorata (Lepidoptera: Noctuidae), is permissive for AcMNPV infection and produces high levels of recombinant proteins
- PMID: 20602790
- PMCID: PMC2906426
- DOI: 10.1186/1472-6750-10-50
Ao38, a new cell line from eggs of the black witch moth, Ascalapha odorata (Lepidoptera: Noctuidae), is permissive for AcMNPV infection and produces high levels of recombinant proteins
Erratum in
-
Correction: BTI-Tnao38, a new cell line derived from Trichoplusia ni, is permissive for AcMNPV infection and produces high levels of recombinant proteins.BMC Biotechnol. 2012 Apr 24;12:12. doi: 10.1186/1472-6750-12-12. BMC Biotechnol. 2012. PMID: 22531032 Free PMC article.
Abstract
Background: The insect cell line is a critical component in the production of recombinant proteins in the baculovirus expression system and new cell lines hold the promise of increasing both quantity and quality of protein production.
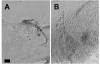
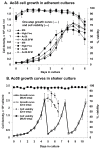
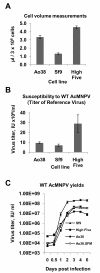
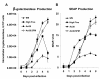
Results: Seventy cell lines were established by single-cell cloning from a primary culture of cells derived from eggs of the black witch moth (Ascalapha odorata; Lepidoptera, Noctuidae). Among 8 rapidly growing lines, cell line 38 (Ao38) was selected for further analysis, based on susceptibility to AcMNPV infection and production of secreted alkaline phosphatase (SEAP) from a baculovirus expression vector. In comparisons with low-passage High Five (BTI-Tn-5B1-4) cells, infected Ao38 cells produced beta-galactosidase and SEAP at levels higher (153% and 150%, respectively) than those measured from High Five cells. Analysis of N-glycans of SEAP produced in Ao38 cells revealed two N-glycosylation sites and glycosylation patterns similar to those reported for High Five and Sf9 cells. Glycopeptide isoforms consisted of pauci- or oligomannose, with and without fucose on N-acetylglucosamine(s) linked to asparagine residues. Estimates of Ao38 cell volume suggest that Ao38 cells are approximately 2.5x larger than Sf9 cells but only approximately 74% of the size of High Five cells. Ao38 cells were highly susceptible to AcMNPV infection, similar to infectivity of Sf9 cells. Production of infectious AcMNPV budded virions from Ao38 cells peaked at approximately 4.5 x 10(7) IU/ml, exceeding that from High Five cells while lower than that from Sf9 cells. Ao38 cells grew rapidly in stationary culture with a population doubling time of 20.2 hr, and Ao38 cells were readily adapted to serum-free medium (Sf-900III) and to a suspension culture system. Analysis of Ao38 and a parental Ascalapha odorata cell line indicated that these lines were free of the alphanodavirus that was recently identified as an adventitious agent in High Five cell lines.
Conclusions: Ao38 cells represent a highly productive new insect cell line that will be useful for heterologous protein expression and other applications in biotechnology.
Figures


References
-
- Strand MR. The insect cellular immune response. Insect Science. 2008;15(1):1–14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

