Mobile interspersed repeats are major structural variants in the human genome
- PMID: 20602999
- PMCID: PMC2943426
- DOI: 10.1016/j.cell.2010.05.026
Mobile interspersed repeats are major structural variants in the human genome
Abstract
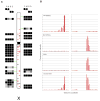
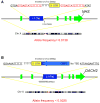
Characterizing structural variants in the human genome is of great importance, but a genome wide analysis to detect interspersed repeats has not been done. Thus, the degree to which mobile DNAs contribute to genetic diversity, heritable disease, and oncogenesis remains speculative. We perform transposon insertion profiling by microarray (TIP-chip) to map human L1(Ta) retrotransposons (LINE-1 s) genome-wide. This identified numerous novel human L1(Ta) insertional polymorphisms with highly variant allelic frequencies. We also explored TIP-chip's usefulness to identify candidate alleles associated with different phenotypes in clinical cohorts. Our data suggest that the occurrence of new insertions is twice as high as previously estimated, and that these repeats are under-recognized as sources of human genomic and phenotypic diversity. We have just begun to probe the universe of human L1(Ta) polymorphisms, and as TIP-chip is applied to other insertions such as Alu SINEs, it will expand the catalog of genomic variants even further.
Figures





Comment in
-
Retrotransposition and structural variation in the human genome.Cell. 2010 Jun 25;141(7):1110-2. doi: 10.1016/j.cell.2010.06.014. Cell. 2010. PMID: 20602993
-
Genomic variation: Human retrotransposons keep it active.Nat Rev Genet. 2010 Aug;11(8):527. doi: 10.1038/nrg2832. Epub 2010 Jul 13. Nat Rev Genet. 2010. PMID: 20628350 No abstract available.
-
Jumping genes.Nat Methods. 2010 Aug;7(8):579. doi: 10.1038/nmeth0810-579. Nat Methods. 2010. PMID: 20704015 No abstract available.
References
-
- Arnold C, Hodgson IJ. Vectorette PCR: A novel approach to genomic walking. PCR Methods Appl. 1991;1:39–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

