Discovery of a proneurogenic, neuroprotective chemical
- PMID: 20603013
- PMCID: PMC2930815
- DOI: 10.1016/j.cell.2010.06.018
Discovery of a proneurogenic, neuroprotective chemical
Abstract
An in vivo screen was performed in search of chemicals capable of enhancing neuron formation in the hippocampus of adult mice. Eight of 1000 small molecules tested enhanced neuron formation in the subgranular zone of the dentate gyrus. Among these was an aminopropyl carbazole, designated P7C3, endowed with favorable pharmacological properties. In vivo studies gave evidence that P7C3 exerts its proneurogenic activity by protecting newborn neurons from apoptosis. Mice missing the gene encoding neuronal PAS domain protein 3 (NPAS3) are devoid of hippocampal neurogenesis and display malformation and electrophysiological dysfunction of the dentate gyrus. Prolonged administration of P7C3 to npas3(-/-) mice corrected these deficits by normalizing levels of apoptosis of newborn hippocampal neurons. Prolonged administration of P7C3 to aged rats also enhanced neurogenesis in the dentate gyrus, impeded neuron death, and preserved cognitive capacity as a function of terminal aging. PAPERCLIP:
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


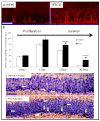





References
-
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sablin S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. - PubMed
-
- Bachurin SO, Shevtosa EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

