Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface
- PMID: 20603080
- PMCID: PMC3178184
- DOI: 10.1016/j.molcel.2010.06.029
Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface
Abstract
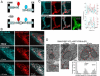
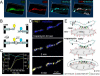
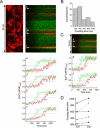
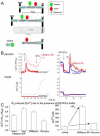
The ER-mitochondrial junction provides a local calcium signaling domain that is critical for both matching energy production with demand and the control of apoptosis. Here, we visualize ER-mitochondrial contact sites and monitor the localized [Ca(2+)] changes ([Ca(2+)](ER-mt)) using drug-inducible fluorescent interorganelle linkers. We show that all mitochondria have contacts with the ER, but plasma membrane (PM)-mitochondrial contacts are less frequent because of interleaving ER stacks in both RBL-2H3 and H9c2 cells. Single mitochondria display discrete patches of ER contacts and show heterogeneity in the ER-mitochondrial Ca(2+) transfer. Pericam-tagged linkers revealed IP(3)-induced [Ca(2+)](ER-mt) signals that exceeded 9 microM and endured buffering bulk cytoplasmic [Ca(2+)] increases. Altering linker length to modify the space available for the Ca(2+) transfer machinery had a biphasic effect on [Ca(2+)](ER-mt) signals. These studies provide direct evidence for the existence of high-Ca(2+) microdomains between the ER and mitochondria and suggest an optimal gap width for efficient Ca(2+) transfer.
2010 Elsevier Inc. All rights reserved.
Figures


Comment in
-
What the mitochondria see.Mol Cell. 2010 Jul 9;39(1):6-7. doi: 10.1016/j.molcel.2010.06.030. Mol Cell. 2010. PMID: 20603071
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

