FGF receptors 1 and 2 control chemically induced injury and compound detoxification in regenerating livers of mice
- PMID: 20603121
- PMCID: PMC2949525
- DOI: 10.1053/j.gastro.2010.06.069
FGF receptors 1 and 2 control chemically induced injury and compound detoxification in regenerating livers of mice
Abstract
Background & aims: Fibroblast growth factor receptor 4 (FGFR4) controls bile acid metabolism and protects the liver from fibrosis, but the roles of FGFR1 and FGFR2 in the adult liver are largely unknown. We investigated the functions and mechanisms of action of these receptors in liver homeostasis, regeneration, and fibrosis.
Methods: We generated mice with hepatocytes that lack FGFR1 and FGFR2 and subjected them to acute and chronic carbon tetrachloride-induced liver injury and partial hepatectomy; mice were also injected with FGF7. We performed histology, histomorphometry, real-time reverse transcription polymerase chain reaction, and immunoblot analyses.
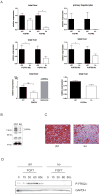
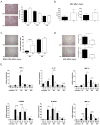
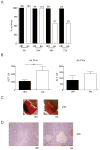
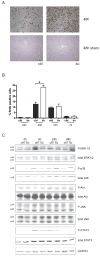
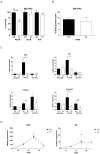
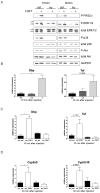
Results: In hepatocytes, loss of FGFR1 and FGFR2 eliminated responsiveness to FGF7 and related FGF family members but did not affect toxin-induced liver injury and fibrosis. However, mortality after partial hepatectomy increased because of severe hepatocyte necrosis. These effects appeared to be mediated by a failure of hepatocytes to induce the expression of the transcriptional regulators Dbp and Tef upon liver surgery; this affected expression of their target genes, which encode detoxifying cytochrome P450 enzymes. We found that Dbp and Tef expression was directly controlled by FGFR signaling in hepatocytes. As a consequence of the reduced expression of genes that control detoxification, the liver tissue that remained after partial hepatectomy failed to efficiently metabolize endogenous compounds and the drugs applied for anesthesia/analgesia.
Conclusions: We identified a new, cytoprotective effect of FGFR1 and FGFR2 in the regenerating liver and suggest the use of recombinant FGF7 to increase survival of patients after surgical resection of large amounts of liver tissue.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. - PubMed
-
- Fausto N. Involvement of the innate immune system in liver regeneration and injury. J Hepatol. 2006;45:347–9. - PubMed
-
- Diehl AM. Liver regeneration. Front Biosci. 2002;7:e301–14. - PubMed
-
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

