A retrospective review of the roles of multifunctional glucose-6-phosphatase in blood glucose homeostasis: Genesis of the tuning/retuning hypothesis
- PMID: 20603134
- PMCID: PMC2937074
- DOI: 10.1016/j.lfs.2010.06.021
A retrospective review of the roles of multifunctional glucose-6-phosphatase in blood glucose homeostasis: Genesis of the tuning/retuning hypothesis
Abstract
In a scientific career spanning from 1955 to 2000, my research focused on phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. Grounded in basic enzymology, and initially pursuing the steady-state rate behavior of isolated preparations of these critically important gluconeogenic enzymes, our key findings were confirmed and extended by in situ enzyme rate experiments exploiting isolated liver perfusions. These efforts culminated in the discovery of the liver cytosolic isozyme of carboxykinase, known today as (GTP)PEPCK-C (EC4.1.1.32) and also revealed a biosynthetic function and multicomponent nature of glucose-6-phosphatase (EC3.1.3.9). Discovery that glucose-6-phosphatase possessed an intrinsically biosynthetic activity, now known as carbamyl-P:glucose phosphotransferase - along with a deeper consideration of the enzyme's hydrolytic activity as well as the action of liver glucokinase resulted in the evolution of Tuning/Retuning Hypothesis for blood glucose homeostasis in health and disease. This THEN & NOW review shares with the reader the joy and exhilaration of major scientific discovery and also contrasts the methodologies and approaches on which I relied with those currently in use.
2010 Elsevier Inc. All rights reserved.
Figures
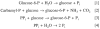





Similar articles
-
The role of hepatic, renal and intestinal gluconeogenic enzymes in glucose homeostasis of juvenile rainbow trout.J Comp Physiol B. 2008 Mar;178(3):429-38. doi: 10.1007/s00360-007-0235-7. Epub 2008 Jan 8. J Comp Physiol B. 2008. PMID: 18180932
-
Dietary and hormonal regulation of some enzyme activities associated with gluconeogenesis in rabbit liver.Biochim Biophys Acta. 1976 Feb 24;421(2):228-36. doi: 10.1016/0304-4165(76)90289-0. Biochim Biophys Acta. 1976. PMID: 175842
-
Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats?Am J Physiol Regul Integr Comp Physiol. 2007 Apr;292(4):R1400-7. doi: 10.1152/ajpregu.00566.2006. Epub 2006 Dec 7. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17158265
-
Novel concepts in insulin regulation of hepatic gluconeogenesis.Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E685-92. doi: 10.1152/ajpendo.00253.2003. Am J Physiol Endocrinol Metab. 2003. PMID: 12959935 Review.
-
Regulation of glucose production by the liver.Annu Rev Nutr. 1999;19:379-406. doi: 10.1146/annurev.nutr.19.1.379. Annu Rev Nutr. 1999. PMID: 10448530 Review.
Cited by
-
Metabolomic heterogeneity of pulmonary arterial hypertension.PLoS One. 2014 Feb 12;9(2):e88727. doi: 10.1371/journal.pone.0088727. eCollection 2014. PLoS One. 2014. PMID: 24533144 Free PMC article.
-
Elevated glucose represses liver glucokinase and induces its regulatory protein to safeguard hepatic phosphate homeostasis.Diabetes. 2011 Dec;60(12):3110-20. doi: 10.2337/db11-0061. Epub 2011 Oct 19. Diabetes. 2011. PMID: 22013014 Free PMC article.
-
Hypothesis: A Novel Neuroprotective Role for Glucose-6-phosphatase (G6PC3) in Brain-To Maintain Energy-Dependent Functions Including Cognitive Processes.Neurochem Res. 2020 Nov;45(11):2529-2552. doi: 10.1007/s11064-020-03113-z. Epub 2020 Aug 19. Neurochem Res. 2020. PMID: 32815045 Review.
-
Histidine Promotes the Glucose Synthesis through Activation of the Gluconeogenic Pathway in Bovine Hepatocytes.Animals (Basel). 2021 Nov 18;11(11):3295. doi: 10.3390/ani11113295. Animals (Basel). 2021. PMID: 34828026 Free PMC article.
-
Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway.Exp Ther Med. 2021 Jan;21(1):53. doi: 10.3892/etm.2020.9485. Epub 2020 Nov 19. Exp Ther Med. 2021. PMID: 33273981 Free PMC article.
References
-
- Alvares FL, Nordlie RC. Quantitative correlation of glucose uptake and phosphorylation with the activities of glucose-phosphorylating enzymes in perfused livers of fasted and fed rats. Journal of Biological Chemistry. 1977;252:8404–8414. - PubMed
-
- Argaud D, Kirby LT, Newgard CB, Lange AJ. Stimulation of glucose-6-phosphatase gene expression by glucose and fructose-2,6-bisphosphate. Journal of Biological Chemistry. 1997;272 (19):12854–12861. - PubMed
-
- Arion WJ, Nordlie RC. Liver microsomal glucose 6-phosphatase, inorganic pyrophosphatase, and pyrophosphate-glucose phosphotransferase ii. Kinetic studies. Journal of Biological Chemistry. 1964;239:2752–2757. - PubMed
-
- Arion WJ, Nordlie RC. Liver glucose-6-phosphatase and pyrophosphate-glucose phosphotransferase: Effects of fasting. Biochemical and Biophysical Research Communications. 1965;20:606–610. - PubMed
-
- Arion WJ, Nordlie RC. Biological regulation of inorganic pyrophosphate-glucose phosphotransferase and glucose 6-phosphate; activation by triamcinolone, in vivo, in the presence of actinomycin d. Journal of Biological Chemistry. 1967;242:2207–2210. - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

