SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model
- PMID: 20603135
- PMCID: PMC2921468
- DOI: 10.1016/j.vaccine.2010.06.073
SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model
Abstract
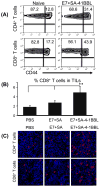
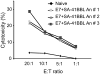
Cervical cancer is the leading cause of cancer-related deaths among women worldwide. Current prophylactic vaccines based on HPV (Human papillomavirus) late gene protein L1 are ineffective in therapeutic settings. Therefore, there is an acute need for the development of therapeutic vaccines for HPV associated cancers. The HPV E7 oncoprotein is expressed in cervical cancer and has been associated with the cellular transformation and maintenance of the transformed phenotype. As such, E7 protein represents an ideal target for the development of therapeutic subunit vaccines against cervical cancer. However, the low antigenicity of this protein may require potent adjuvants for therapeutic efficacy. We recently generated a novel chimeric form of the 4-1BBL costimulatory molecule engineered with core streptavidin (SA-4-1BBL) and demonstrated its safe and pleiotropic effects on various cells of the immune system. We herein tested the utility of SA-4-1BBL as the immunomodulatory component of HPV-16 E7 recombinant protein based therapeutic vaccine in the E7 expressing TC-1 tumor as a model of cervical cancer in mice. A single subcutaneous vaccination was effective in eradicating established tumors in approximately 70% of mice. The therapeutic efficacy of the vaccine was associated with robust primary and memory CD4(+) and CD8(+) T cell responses, Th1 cytokine response, infiltration of CD4(+) and CD8(+) T cells into the tumor, and enhanced NK cell killing. Importantly, NK cells played an important role in vaccine mediated therapy since their physical depletion compromised vaccine efficacy. Collectively, these data demonstrate the utility of SA-4-1BBL as a new class of multifunctional immunomodulator for the development of therapeutic vaccines against cancer and chronic infections.
Conflict of interest statement
Figures
Similar articles
-
A Genetically Modified attenuated Listeria Vaccine Expressing HPV16 E7 Kill Tumor Cells in Direct and Antigen-Specific Manner.Front Cell Infect Microbiol. 2017 Jun 29;7:279. doi: 10.3389/fcimb.2017.00279. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28706878 Free PMC article.
-
Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody.mBio. 2021 Jan 19;12(1):e03224-20. doi: 10.1128/mBio.03224-20. mBio. 2021. PMID: 33468698 Free PMC article.
-
4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines.Cancer Res. 2010 May 15;70(10):3945-54. doi: 10.1158/0008-5472.CAN-09-4480. Epub 2010 Apr 20. Cancer Res. 2010. PMID: 20406989 Free PMC article.
-
Genetically modified cellular vaccines for therapy of human papilloma virus type 16 (HPV 16)-associated tumours.Curr Cancer Drug Targets. 2008 May;8(3):180-6. doi: 10.2174/156800908784293596. Curr Cancer Drug Targets. 2008. PMID: 18473731 Review.
-
The current state of therapeutic and T cell-based vaccines against human papillomaviruses.Virus Res. 2017 Mar 2;231:148-165. doi: 10.1016/j.virusres.2016.12.002. Epub 2016 Dec 6. Virus Res. 2017. PMID: 27932207 Free PMC article. Review.
Cited by
-
Human Papillomavirus Type 16 Disables the Increased Natural Killer Cells in Early Lesions of the Cervix.J Immunol Res. 2019 Apr 28;2019:9182979. doi: 10.1155/2019/9182979. eCollection 2019. J Immunol Res. 2019. PMID: 31183395 Free PMC article.
-
Targeting of the tumor necrosis factor receptor superfamily for cancer immunotherapy.ISRN Oncol. 2013 Jun 11;2013:371854. doi: 10.1155/2013/371854. Print 2013. ISRN Oncol. 2013. PMID: 23840967 Free PMC article.
-
SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines.Expert Rev Vaccines. 2014 Mar;13(3):387-98. doi: 10.1586/14760584.2014.880340. Expert Rev Vaccines. 2014. PMID: 24521311 Free PMC article. Review.
-
Formulation and evaluation of oral microparticulate ovarian cancer vaccines.Vaccine. 2012 Aug 17;30(38):5675-81. doi: 10.1016/j.vaccine.2012.05.073. Epub 2012 Jun 28. Vaccine. 2012. PMID: 22750042 Free PMC article.
-
A novel agonist of 4-1BB costimulatory receptor shows therapeutic efficacy against a tobacco carcinogen-induced lung cancer.Cancer Immunol Immunother. 2023 Nov;72(11):3567-3579. doi: 10.1007/s00262-023-03507-2. Epub 2023 Aug 22. Cancer Immunol Immunother. 2023. PMID: 37605009 Free PMC article.
References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. - PubMed
-
- Brandsma JL, Abramson AL. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989;115:621–5. - PubMed
-
- Schmiedeskamp MR, Kockler DR. Human papillomavirus vaccines. Ann Pharmacother. 2006;40:1344–52. - PubMed
-
- Monie A, Hung CF, Wu TC. Preventive and therapeutic HPV vaccines. Curr Opin Investig Drugs. 2007;8:1038–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

