The SH2 domain protein Shep1 regulates the in vivo signaling function of the scaffolding protein Cas
- PMID: 20603213
- PMCID: PMC2948029
- DOI: 10.1016/j.cellsig.2010.06.015
The SH2 domain protein Shep1 regulates the in vivo signaling function of the scaffolding protein Cas
Abstract
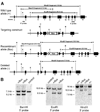
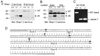
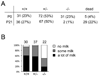
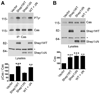
The members of the p130Cas (Cas) family are important scaffolding proteins that orchestrate cell adhesion, migration and invasiveness downstream of integrin adhesion receptors and receptor tyrosine kinases by recruiting enzymes and structural molecules. Shep1, BCAR3/AND-34 and NSP1 define a recently identified family of SH2 domain-containing proteins that constitutively bind Cas proteins through a Cdc25-type nucleotide exchange factor-like domain. To gain insight into the functional interplay between Shep1 and Cas in vivo, we have inactivated the Shep1 gene in the mouse through Cre-mediated deletion of the exon encoding the SH2 domain. Analysis of Cas tyrosine phosphorylation in the brains of newborn mice, where Shep1 is highly expressed, revealed a strong decrease in Cas substrate domain phosphorylation in knockout compared to wild-type brains. Src family kinases bind to Cas via their SH3 and SH2 domains, which contributes to their activation, and phosphorylate multiple tyrosines in the Cas substrate domain. These tyrosine-phosphorylated motifs represent docking sites for the Crk adaptor, linking Cas to the downstream Rac1 and Rap1 GTPases to regulate cell adhesion and actin cytoskeleton organization. Accordingly, we detected lower Cas-Crk association and lower phosphorylation of the Src activation loop in Shep1 knockout brains compared to wild-type. Conversely, Shep1 transfection in COS cells increases Cas tyrosine phosphorylation. The SH2 domain is likely critical for the effects of Shep1 on Cas and Src signaling because the knockout mice express Shep1 fragments that lack the amino-terminal region including the SH2 domain, presumably due to aberrant translation from internal ATG codons. These fragments retain the ability to increase Cas levels in transfected cells, similar to full-length Shep1. However, they do not affect Cas phosphorylation on their own or in the presence of co-transfected full-length Shep1. They also do not show dominant negative effects on the activity of full-length Shep1 in vivo because the heterozygous mice, which express the fragments, have a normal life span. This is in contrast to the homozygous knockout mice, most of which die soon after birth. These data demonstrate that Shep1 plays a critical role in the in vivo regulation of Src activity and Cas downstream signaling through Crk, and suggest that the SH2 domain of Shep1 is critical for these effects.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Breast cancer anti-estrogen resistance 3 (BCAR3) protein augments binding of the c-Src SH3 domain to Crk-associated substrate (p130cas).J Biol Chem. 2012 Aug 10;287(33):27703-14. doi: 10.1074/jbc.M112.389981. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711540 Free PMC article.
-
SHEP1 function in cell migration is impaired by a single amino acid mutation that disrupts association with the scaffolding protein cas but not with Ras GTPases.J Biol Chem. 2004 Oct 1;279(40):41892-902. doi: 10.1074/jbc.M402929200. Epub 2004 Jul 22. J Biol Chem. 2004. PMID: 15272013
-
Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases.Mol Cell Biol. 1996 Jun;16(6):2606-13. doi: 10.1128/MCB.16.6.2606. Mol Cell Biol. 1996. PMID: 8649368 Free PMC article.
-
Crk family adaptors-signalling complex formation and biological roles.Oncogene. 2001 Oct 1;20(44):6348-71. doi: 10.1038/sj.onc.1204779. Oncogene. 2001. PMID: 11607838 Review.
-
The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL.Int J Mol Sci. 2025 Apr 24;26(9):4017. doi: 10.3390/ijms26094017. Int J Mol Sci. 2025. PMID: 40362257 Free PMC article. Review.
Cited by
-
NSP-CAS Protein Complexes: Emerging Signaling Modules in Cancer.Genes Cancer. 2012 May;3(5-6):382-93. doi: 10.1177/1947601912460050. Genes Cancer. 2012. PMID: 23226576 Free PMC article.
-
A Cas-BCAR3 co-regulatory circuit controls lamellipodia dynamics.Elife. 2021 Jun 25;10:e67078. doi: 10.7554/eLife.67078. Elife. 2021. PMID: 34169835 Free PMC article.
-
SHEP1 partners with CasL to promote marginal zone B-cell maturation.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18944-9. doi: 10.1073/pnas.1007558107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956287 Free PMC article.
-
Profiling, Bioinformatic, and Functional Data on the Developing Olfactory/GnRH System Reveal Cellular and Molecular Pathways Essential for This Process and Potentially Relevant for the Kallmann Syndrome.Front Endocrinol (Lausanne). 2013 Dec 31;4:203. doi: 10.3389/fendo.2013.00203. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 24427155 Free PMC article.
-
The SRC homology 2 domain protein Shep1 plays an important role in the penetration of olfactory sensory axons into the forebrain.J Neurosci. 2010 Sep 29;30(39):13201-10. doi: 10.1523/JNEUROSCI.3289-10.2010. J Neurosci. 2010. PMID: 20881139 Free PMC article.
References
-
- O'Neill GM, Fashena SJ, Golemis EA. Trends Cell Biol. 2000;10(3):111–119. - PubMed
-
- Bouton AH, Riggins RB, Bruce-Staskal PJ. Oncogene. 2001;20(44):6448–6458. - PubMed
-
- Defilippi P, Di Stefano P, Cabodi S. Trends Cell Biol. 2006;16(5):257–263. - PubMed
-
- Feller SM. Journal of Cellular Physiology. 1998;177(4):535–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

