Designing and implementing sample and data collection for an international genetics study: the Type 1 Diabetes Genetics Consortium (T1DGC)
- PMID: 20603248
- PMCID: PMC2917852
- DOI: 10.1177/1740774510373497
Designing and implementing sample and data collection for an international genetics study: the Type 1 Diabetes Genetics Consortium (T1DGC)
Abstract
Background and purpose: The Type 1 Diabetes Genetics Consortium (T1DGC) is an international project whose primary aims are to: (a) discover genes that modify type 1 diabetes risk; and (b) expand upon the existing genetic resources for type 1 diabetes research. The initial goal was to collect 2500 affected sibling pair (ASP) families worldwide.
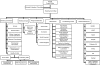
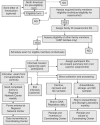
Methods: T1DGC was organized into four regional networks (Asia-Pacific, Europe, North America, and the United Kingdom) and a Coordinating Center. A Steering Committee, with representatives from each network, the Coordinating Center, and the funding organizations, was responsible for T1DGC operations. The Coordinating Center, with regional network representatives, developed study documents and data systems. Each network established laboratories for: DNA extraction and cell line production; human leukocyte antigen genotyping; and autoantibody measurement. Samples were tracked from the point of collection, processed at network laboratories and stored for deposit at National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repositories. Phenotypic data were collected and entered into the study database maintained by the Coordinating Center.
Results: T1DGC achieved its original ASP recruitment goal. In response to research design changes, the T1DGC infrastructure also recruited trios, cases, and controls. Results of genetic analyses have identified many novel regions that affect susceptibility to type 1 diabetes. T1DGC created a resource of data and samples that is accessible to the research community.
Limitations: Participation in T1DGC was declined by some countries due to study requirements for the processing of samples at network laboratories and/or final deposition of samples in NIDDK Central Repositories. Re-contact of participants was not included in informed consent templates, preventing collection of additional samples for functional studies.
Conclusions: T1DGC implemented a distributed, regional network structure to reach ASP recruitment targets. The infrastructure proved robust and flexible enough to accommodate additional recruitment. T1DGC has established significant resources that provide a basis for future discovery in the study of type 1 diabetes genetics.
Figures
Similar articles
-
Collection and processing of whole blood for transformation of peripheral blood mononuclear cells and extraction of DNA: the Type 1 Diabetes Genetics Consortium.Clin Trials. 2010;7(1 Suppl):S65-74. doi: 10.1177/1740774510373493. Epub 2010 Jul 1. Clin Trials. 2010. PMID: 20595244 Free PMC article.
-
Design and Measurement of Nonislet-Specific Autoantibodies for the Type 1 Diabetes Genetics Consortium Autoantibody Workshop.Diabetes Care. 2015 Oct;38 Suppl 2(Suppl 2):S4-7. doi: 10.2337/dcs15-2002. Diabetes Care. 2015. PMID: 26405071 Free PMC article.
-
Role of Type 1 Diabetes-Associated SNPs on Autoantibody Positivity in the Type 1 Diabetes Genetics Consortium: Overview.Diabetes Care. 2015 Oct;38 Suppl 2(Suppl 2):S1-3. doi: 10.2337/dcs15-2001. Diabetes Care. 2015. PMID: 26405066 Free PMC article.
-
Type 1 Diabetes Genetics Consortium.J Clin Endocrinol Metab. 2025 May 19;110(6):1505-1513. doi: 10.1210/clinem/dgaf181. J Clin Endocrinol Metab. 2025. PMID: 40117445 Free PMC article. Review.
-
The Type 1 Diabetes Genetics Consortium.Ann N Y Acad Sci. 2006 Oct;1079:1-8. doi: 10.1196/annals.1375.001. Ann N Y Acad Sci. 2006. PMID: 17130525 Review.
Cited by
-
Collection and processing of whole blood for transformation of peripheral blood mononuclear cells and extraction of DNA: the Type 1 Diabetes Genetics Consortium.Clin Trials. 2010;7(1 Suppl):S65-74. doi: 10.1177/1740774510373493. Epub 2010 Jul 1. Clin Trials. 2010. PMID: 20595244 Free PMC article.
-
Association Between Schizophrenia-Related Polygenic Liability and the Occurrence and Level of Mood-Incongruent Psychotic Symptoms in Bipolar Disorder.JAMA Psychiatry. 2018 Jan 1;75(1):28-35. doi: 10.1001/jamapsychiatry.2017.3485. JAMA Psychiatry. 2018. PMID: 29167880 Free PMC article.
-
Monogenic Causes in the Type 1 Diabetes Genetics Consortium Cohort: Low Genetic Risk for Autoimmunity in Case Selection.J Clin Endocrinol Metab. 2021 May 13;106(6):1804-1810. doi: 10.1210/clinem/dgab056. J Clin Endocrinol Metab. 2021. PMID: 33538814 Free PMC article.
-
Regulation of inflammation in diabetes: From genetics to epigenomics evidence.Mol Metab. 2020 Nov;41:101041. doi: 10.1016/j.molmet.2020.101041. Epub 2020 Jun 27. Mol Metab. 2020. PMID: 32603690 Free PMC article. Review.
-
HLA genotyping in the international Type 1 Diabetes Genetics Consortium.Clin Trials. 2010;7(1 Suppl):S75-87. doi: 10.1177/1740774510373494. Epub 2010 Jul 1. Clin Trials. 2010. PMID: 20595243 Free PMC article.
References
-
- The TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007; 8: 286–98 - PubMed
-
- Hagopian WA, Lernmark A, Rewers MJ, et al. TEDDY – The Environmental Determinants of Diabetes in the Young. Ann NY Acad Sci 2006; 1079: 320–26 - PubMed
-
- Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet natural history study of the development of type 1 diabetes: objective, design and initial results. Pediatr Diabetes 2009; 10: 97–104 - PubMed
-
- Rotrosen D, Matthews JB, Bluestone JA. The immune tolerance network: a new paradigm for developing tolerance-inducing therapies. J Allergy Clin Immunol 2002; 110: 17–23 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical