Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella
- PMID: 20603313
- PMCID: PMC2916133
- DOI: 10.1084/jem.20100257
Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella
Abstract
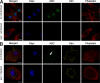
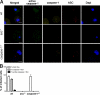
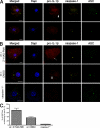
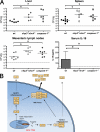
Intracellular pathogens and endogenous danger signals in the cytosol engage NOD-like receptors (NLRs), which assemble inflammasome complexes to activate caspase-1 and promote the release of proinflammatory cytokines IL-1beta and IL-18. However, the NLRs that respond to microbial pathogens in vivo are poorly defined. We show that the NLRs NLRP3 and NLRC4 both activate caspase-1 in response to Salmonella typhimurium. Responding to distinct bacterial triggers, NLRP3 and NLRC4 recruited ASC and caspase-1 into a single cytoplasmic focus, which served as the site of pro-IL-1beta processing. Consistent with an important role for both NLRP3 and NLRC4 in innate immune defense against S. typhimurium, mice lacking both NLRs were markedly more susceptible to infection. These results reveal unexpected redundancy among NLRs in host defense against intracellular pathogens in vivo.
Figures
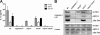

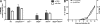
References
-
- Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 30:556–565 10.1016/j.immuni.2009.02.005 - DOI - PMC - PubMed
-
- Amer A., Franchi L., Kanneganti T.D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281:35217–35223 10.1074/jbc.M604933200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

