A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys
- PMID: 20603332
- PMCID: PMC3039262
- DOI: 10.1113/jphysiol.2010.190538
A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys
Abstract
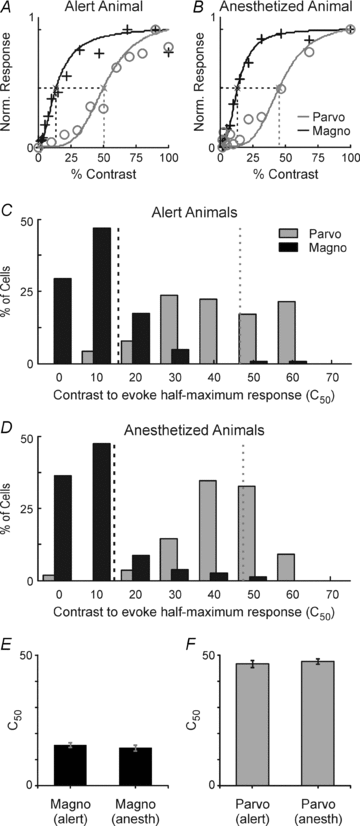
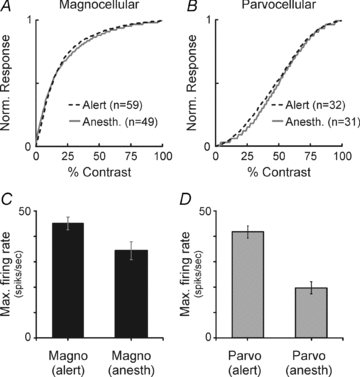
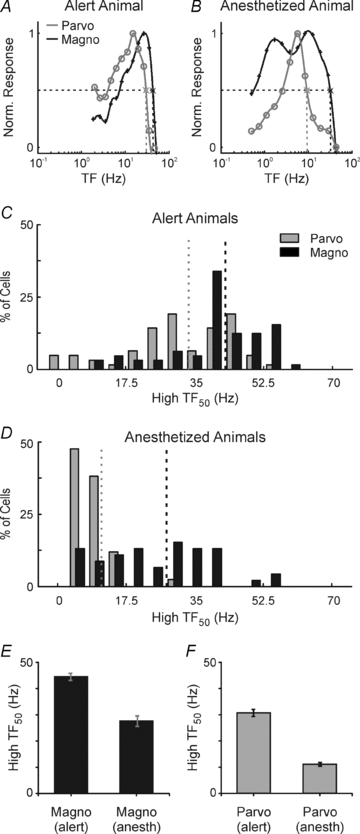
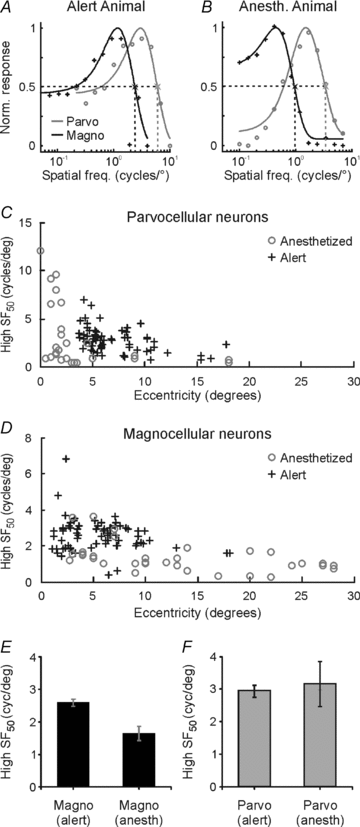
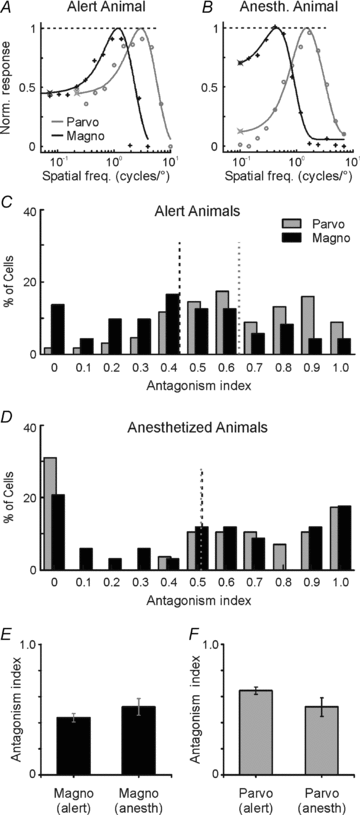
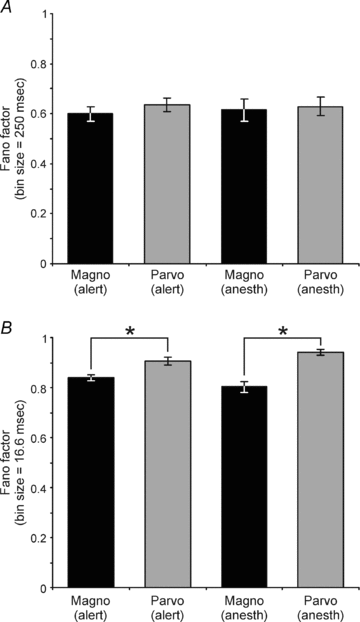
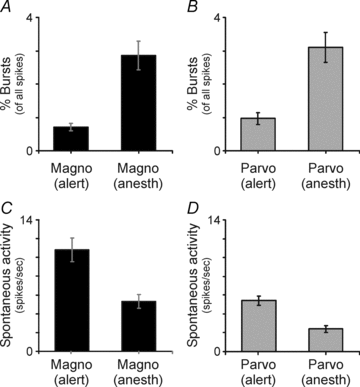
Despite the increasing use of alert animals for studies aimed at understanding visual processing in the cerebral cortex, relatively little attention has been focused on quantifying the response properties of neurons that provide input to the cortex. Here, we examine the response properties of neurons in the lateral geniculate nucleus (LGN) of the thalamus in the alert macaque monkey and compare these responses to those in the anaesthetized animal. Compared to the anaesthetized animal, we show that magnocellular and parvocellular neurons in the alert animal respond to visual stimuli with significantly higher firing rates. This increase in responsiveness is not accompanied by a change in the shape of neuronal contrast response functions or the strength of centre–surround antagonism; however, it is accompanied by an increased ability of neurons to follow stimuli drifting at higher spatial and temporal frequencies.
Figures

References
-
- Ahmad A, Spear PD. Effects of aging on the size, density, and number of rhesus monkey lateral geniculate neurons. J Comp Neurol. 1993;334:631–643. - PubMed
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
-
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Visual Neurosci. 1992;8:483–486. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

