Beyond the wiring diagram: signalling through complex neuromodulator networks
- PMID: 20603357
- PMCID: PMC2894954
- DOI: 10.1098/rstb.2010.0105
Beyond the wiring diagram: signalling through complex neuromodulator networks
Abstract
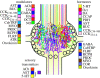
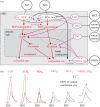
During the computations performed by the nervous system, its 'wiring diagram'--the map of its neurons and synaptic connections--is dynamically modified and supplemented by multiple actions of neuromodulators that can be so complex that they can be thought of as constituting a biochemical network that combines with the neuronal network to perform the computation. Thus, the neuronal wiring diagram alone is not sufficient to specify, and permit us to understand, the computation that underlies behaviour. Here I review how such modulatory networks operate, the problems that their existence poses for the experimental study and conceptual understanding of the computations performed by the nervous system, and how these problems may perhaps be solved and the computations understood by considering the structural and functional 'logic' of the modulatory networks.
Figures

References
-
- Abbracchio M. P., Burnstock G., Verkhratsky A., Zimmermann H.2008Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29 (doi:10.1016/j.tins.2008.10.001) - DOI - PubMed
-
- Agnati L. F., Zoli M., Strömberg I., Fuxe K.1995Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69, 711–726 (doi:10.1016/0306-4522(95)00308-6) - DOI - PubMed
-
- Alon U.2007An introduction to systems biology: design principles of biological circuits Boca Raton, FL: Chapman & Hall/CRC
-
- Alon U., Surette M. G., Barkai N., Leibler S.1999Robustness in bacterial chemotaxis. Nature 397, 168–171 (doi:10.1038/16483) - DOI - PubMed
-
- Baraban S. C., Tallent M. K.2004Interneuronal neuropeptides—endogenous regulators of neuronal excitability. Trends Neurosci. 27, 135–142 (doi:10.1016/j.tins.2004.01.008) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

