Shining light into the black box of spinal locomotor networks
- PMID: 20603359
- PMCID: PMC2894950
- DOI: 10.1098/rstb.2009.0322
Shining light into the black box of spinal locomotor networks
Abstract
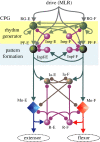
Rhythmic activity is responsible for numerous essential motor functions including locomotion, breathing and chewing. In the case of locomotion, it has been realized for some time that the spinal cord contains sufficient circuitry to produce a sophisticated stepping pattern. However, the central pattern generator for locomotion in mammals has remained a 'black box' where inputs to the network were manipulated and the outputs interpreted. Over the last decade, new genetic approaches and techniques have been developed that provide ways to identify and manipulate the activity of classes of interneurons. The use of these techniques will be critically discussed and related to current models of network function.
Figures

Similar articles
-
Recent Insights into the Rhythmogenic Core of the Locomotor CPG.Int J Mol Sci. 2021 Jan 30;22(3):1394. doi: 10.3390/ijms22031394. Int J Mol Sci. 2021. PMID: 33573259 Free PMC article. Review.
-
Optogenetic Activation of V1 Interneurons Reveals the Multimodality of Spinal Locomotor Networks in the Neonatal Mouse.J Neurosci. 2021 Oct 13;41(41):8545-8561. doi: 10.1523/JNEUROSCI.0875-21.2021. Epub 2021 Aug 26. J Neurosci. 2021. PMID: 34446573 Free PMC article.
-
Genetic dissection of rhythmic motor networks in mice.Prog Brain Res. 2010;187:19-37. doi: 10.1016/B978-0-444-53613-6.00002-2. Prog Brain Res. 2010. PMID: 21111198 Free PMC article.
-
Delineating the Diversity of Spinal Interneurons in Locomotor Circuits.J Neurosci. 2017 Nov 8;37(45):10835-10841. doi: 10.1523/JNEUROSCI.1829-17.2017. J Neurosci. 2017. PMID: 29118212 Free PMC article. Review.
-
Excitatory components of the mammalian locomotor CPG.Brain Res Rev. 2008 Jan;57(1):56-63. doi: 10.1016/j.brainresrev.2007.07.002. Epub 2007 Nov 7. Brain Res Rev. 2008. PMID: 17988744 Review.
Cited by
-
Modelling genetic reorganization in the mouse spinal cord affecting left-right coordination during locomotion.J Physiol. 2013 Nov 15;591(22):5491-508. doi: 10.1113/jphysiol.2013.261115. Epub 2013 Sep 30. J Physiol. 2013. PMID: 24081162 Free PMC article.
-
Neuronal network analyses: premises, promises and uncertainties.Philos Trans R Soc Lond B Biol Sci. 2010 Aug 12;365(1551):2315-28. doi: 10.1098/rstb.2010.0043. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20603354 Free PMC article. Review.
-
Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks.Curr Biol. 2015 Jun 1;25(11):1426-36. doi: 10.1016/j.cub.2015.04.005. Epub 2015 May 7. Curr Biol. 2015. PMID: 25959968 Free PMC article.
-
Neuronal Population Activity in Spinal Motor Circuits: Greater Than the Sum of Its Parts.Front Neural Circuits. 2017 Dec 19;11:103. doi: 10.3389/fncir.2017.00103. eCollection 2017. Front Neural Circuits. 2017. PMID: 29311842 Free PMC article. Review.
-
Abundance of gap junctions at glutamatergic mixed synapses in adult Mosquitofish spinal cord neurons.Front Neural Circuits. 2014 Jun 26;8:66. doi: 10.3389/fncir.2014.00066. eCollection 2014. Front Neural Circuits. 2014. PMID: 25018700 Free PMC article.
References
-
- Airan R. D., Hu E. S., Vijaykumar R., Roy M., Meltzer L. A., Deisseroth K.2007Integration of light-controlled neuronal firing and fast circuit imaging. Curr. Opin. Neurobiol. 17, 587–592 (doi:10.1016/j.conb.2007.11.003) - DOI - PMC - PubMed
-
- Al-Mosawie A., Wilson J. M., Brownstone R. M.2007Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur. J. Neurosci. 26, 3003–3015 (doi:10.1111/j.1460-9568.2007.05907.x) - DOI - PubMed
-
- Alvarez F. J., Jonas P. C., Sapir T., Hartley R., Berrocal M. C., Geiman E. J., Todd A. J., Goulding M.2005Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 493, 177–192 (doi:10.1002/cne.20711) - DOI - PMC - PubMed
-
- Bannatyne B. A., Edgley S. A., Hammar I., Jankowska E., Maxwell D. J.2003Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur. J. Neurosci. 18, 2273–2284 (doi:10.1046/j.1460-9568.2003.02973.x) - DOI - PMC - PubMed
-
- Barbeau H., Rossignol S.1987Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95 (doi:10.1016/0006-8993(87)91442-9) - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

