21 years of biologically effective dose
- PMID: 20603408
- PMCID: PMC3473681
- DOI: 10.1259/bjr/31372149
21 years of biologically effective dose
Abstract
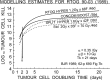
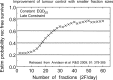
In 1989 the British Journal of Radiology published a review proposing the term biologically effective dose (BED), based on linear quadratic cell survival in radiobiology. It aimed to indicate quantitatively the biological effect of any radiotherapy treatment, taking account of changes in dose-per-fraction or dose rate, total dose and (the new factor) overall time. How has it done so far? Acceptable clinical results have been generally reported using BED, and it is in increasing use, although sometimes mistaken for "biologically equivalent dose", from which it differs by large factors, as explained here. The continuously bending nature of the linear quadratic curve has been questioned but BED has worked well for comparing treatments in many modalities, including some with large fractions. Two important improvements occurred in the BED formula. First, in 1999, high linear energy transfer (LET) radiation was included; second, in 2003, when time parameters for acute mucosal tolerance were proposed, optimum overall times could then be "triangulated" to optimise tumour BED and cell kill. This occurs only when both early and late BEDs meet their full constraints simultaneously. New methods of dose delivery (intensity modulated radiation therapy, stereotactic body radiation therapy, protons, tomotherapy, rapid arc and cyberknife) use a few large fractions and obviously oppose well-known fractionation schedules. Careful biological modelling is required to balance the differing trends of fraction size and local dose gradient, as explained in the discussion "How Fractionation Really Works". BED is now used for dose escalation studies, radiochemotherapy, brachytherapy, high-LET particle beams, radionuclide-targeted therapy, and for quantifying any treatments using ionising radiation.
Figures




Comment in
-
The BJR and progress in radiobiological modelling.Br J Radiol. 2010 Jul;83(991):544-5. doi: 10.1259/bjr/52885245. Br J Radiol. 2010. PMID: 20603406 Free PMC article. No abstract available.
References
-
- Fowler JF. A Review: The linear quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679–675 - PubMed
-
- Park CS, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:847–52 - PubMed
-
- Maciejewski B, Withers HR, Taylor JMG, et al. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx: tumor dose-response and repopulation. Int J Radiat Oncol Biol Phys 1989;16:831–43 - PubMed
-
- Curtis SB. Lethal and potentially lethal lesions induced by radiation – a unified repair model. Radiat Res 1986;106:252–70 - PubMed
-
- Gilbert CW, Hendry JH, Major D. The approximation in the formulation for survival of S = exp – (αd+βd2). Int J Radiat Biol;37:469–71 - PubMed
References to the appendices
-
- Dale RG, Jones B. The assessment of RBE effects using the concept of biologically effective dose. Int J Radiat Oncol Biol Phys 1999;43:639–45 - PubMed
-
- Dale RG, Carabe-Ferandez A. the radiobiology of conventional radiotherapy and its application to radionuclide therapy. Cancer Biother Radiopharm 2005;20:47–51 - PubMed
-
- Carabe-Fernandez A, Dale RG, Jones B. The incorporation of the concept of minimum RBE (RBEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int J Radiat Biol 2007;83:27–39 - PubMed
-
- Jones B, Carabe-Fernandez A, Dale RG. Calculation of high-LET radiotherapy dose required for compensation of overall treatment time extensions. Br J Radiol 2005;79:254–7 - PubMed
-
- Dale RG, Jones B, Coles IP. The effect of tumour shrinkage on the biological effectiveness of permanent brachytherapy implants. Br J Radiol 1994;67:639–45 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

