Molecular cloning and characterization of mitogen-activated protein kinase 2 in Trypanosoma cruzi
- PMID: 20603604
- PMCID: PMC3040964
- DOI: 10.4161/cc.9.14.12372
Molecular cloning and characterization of mitogen-activated protein kinase 2 in Trypanosoma cruzi
Abstract
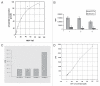
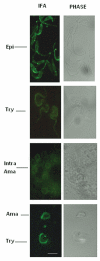
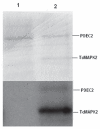
Mitogen-activated protein kinase (MAPK) pathways are major signal transduction systems by which eukaryotic cells convert environmental cues to intracellular events such as proliferation and differentiation. We have identified a Trypanosoma cruzi homologue of the MAPK family that we have called TcMAPK2. Sequence analyses demonstrates TcMAPK2 has high homology with lower eukaryotic ERK2 but has significant differences from mammalian ERK2. Enzymatic assays of both recombinant TcMAPK2 and native protein obtained by immunoprecipitation using anti-TcMAPK2 demonstrated that both preparations of TcMAPK2 were catalytically active. Immunofluorescence analysis of the subcellular localization of TcMAPK2 determined it is mainly cytoplasmic in epimastigotes, along the flagella in trypomastigotes and on the plasma membrane of intracellular amastigotes. Phosphorylated TcMAPK2 was highest in trypomastigotes and lowest in amastigotes. Recombinant TcMAPK2 was able to phosphorylate the recombinant protein of a cAMP specific phosphodiesterase. Overexpression of TcMAPK2 in epimastigotes inhibited growth and development leading to death. TcMAPK2 has an important role in the stress response of the parasite and may be important in regulating proliferation and differentiation.
Figures



Similar articles
-
Molecular cloning and characterization of mitogen-activated protein kinase 2 in Toxoplasma gondii.Cell Cycle. 2011 Oct 15;10(20):3519-26. doi: 10.4161/cc.10.20.17791. Epub 2011 Oct 15. Cell Cycle. 2011. PMID: 22030559 Free PMC article.
-
Molecular cloning of a Trypanosoma cruzi cell surface casein kinase II substrate, Tc-1, involved in cellular infection.Infect Immun. 2006 Jul;74(7):3922-9. doi: 10.1128/IAI.00045-06. Infect Immun. 2006. PMID: 16790765 Free PMC article.
-
Potential involvement of extracellular signal-regulated kinase 1 and 2 in encystation of a primitive eukaryote, Giardia lamblia. Stage-specific activation and intracellular localization.J Biol Chem. 2003 Jan 17;278(3):1936-45. doi: 10.1074/jbc.M209274200. Epub 2002 Oct 22. J Biol Chem. 2003. PMID: 12397063
-
Cloning of a cdc2-related protein kinase from Trypanosoma cruzi that interacts with mammalian cyclins.Mol Biochem Parasitol. 1998 Mar 15;91(2):337-51. doi: 10.1016/s0166-6851(97)00218-1. Mol Biochem Parasitol. 1998. PMID: 9580532
-
Signal transduction in Trypanosoma cruzi.Adv Parasitol. 2011;75:325-44. doi: 10.1016/B978-0-12-385863-4.00015-0. Adv Parasitol. 2011. PMID: 21820563 Review.
Cited by
-
Golgi UDP-GlcNAc:polypeptide O-α-N-Acetyl-d-glucosaminyltransferase 2 (TcOGNT2) regulates trypomastigote production and function in Trypanosoma cruzi.Eukaryot Cell. 2014 Oct;13(10):1312-27. doi: 10.1128/EC.00165-14. Epub 2014 Aug 1. Eukaryot Cell. 2014. PMID: 25084865 Free PMC article.
-
Enzyme Activity Assays for Protein Kinases: Strategies to Identify Active Substrates.Curr Drug Discov Technol. 2016;13(1):2-15. doi: 10.2174/1570163813666160115125930. Curr Drug Discov Technol. 2016. PMID: 26768716 Free PMC article. Review.
-
cAMP signalling in trypanosomatids: role in pathogenesis and as a drug target.Trends Parasitol. 2015 Aug;31(8):373-9. doi: 10.1016/j.pt.2015.04.014. Epub 2015 May 21. Trends Parasitol. 2015. PMID: 26004537 Free PMC article. Review.
-
Adhesion of Trypanosoma cruzi trypomastigotes to fibronectin or laminin modifies tubulin and paraflagellar rod protein phosphorylation.PLoS One. 2012;7(10):e46767. doi: 10.1371/journal.pone.0046767. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056443 Free PMC article.
-
Molecular cloning and characterization of mitogen-activated protein kinase 2 in Toxoplasma gondii.Cell Cycle. 2011 Oct 15;10(20):3519-26. doi: 10.4161/cc.10.20.17791. Epub 2011 Oct 15. Cell Cycle. 2011. PMID: 22030559 Free PMC article.
References
-
- Naula C, Seebeck T. Cyclic AMP Signaling in Trypanosomatides. Parasitol Today. 2000;16:35–38. - PubMed
-
- Tomlinson S, Vandekerckhove F, Frevert U, Nussenzweig V. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitol. 1995;110:547–554. - PubMed
-
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
