Cell adhesive behavior on thin polyelectrolyte multilayers: cells attempt to achieve homeostasis of its adhesion energy
- PMID: 20604583
- PMCID: PMC2918384
- DOI: 10.1021/la101689z
Cell adhesive behavior on thin polyelectrolyte multilayers: cells attempt to achieve homeostasis of its adhesion energy
Abstract
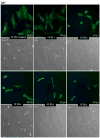
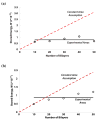
Linearly growing ultrathin polyelectrolyte multilayer (PEM) films of strong polyelectrolytes, poly(diallyldimethylammonium chloride) (PDAC), and sulfonated polystyrene, sodium salt (SPS) exhibit a gradual shift from cytophilic to cytophobic behavior, with increasing thickness for films of less than 100 nm. Previous explanations based on film hydration, swelling, and changes in the elastic modulus cannot account for the cytophobicity observed with these thin films as the number of bilayers increases. We implemented a finite element analysis to help elucidate the observed trends in cell spreading. The simulation results suggest that cells maintain a constant level of energy consumption (energy homeostasis) during active probing and thus respond to changes in the film stiffness as the film thickness increases by adjusting their morphology and the number of focal adhesions recruited and thereby their attachment to a substrate.
Figures






Similar articles
-
Tunable swelling of polyelectrolyte multilayers in cell culture media for modulating NIH-3T3 cells adhesion.J Biomed Mater Res A. 2014 Nov;102(11):4071-7. doi: 10.1002/jbm.a.35094. Epub 2014 Feb 4. J Biomed Mater Res A. 2014. PMID: 24470104
-
Polyelectrolytes Multilayers to Modulate Cell Adhesion: A Study of the Influence of Film Composition and Polyelectrolyte Interdigitation on the Adhesion of the A549 Cell Line.Macromol Biosci. 2016 Apr;16(4):482-95. doi: 10.1002/mabi.201500275. Epub 2015 Dec 11. Macromol Biosci. 2016. PMID: 26663657
-
Cell Adhesion and Proliferation on the "Living" Surface of a Polyelectrolyte Multilayer.Langmuir. 2016 May 31;32(21):5412-21. doi: 10.1021/acs.langmuir.6b00784. Epub 2016 May 18. Langmuir. 2016. PMID: 27191244
-
Controlling primary hepatocyte adhesion and spreading on protein-free polyelectrolyte multilayer films.J Am Chem Soc. 2004 Dec 22;126(50):16286-7. doi: 10.1021/ja046188u. J Am Chem Soc. 2004. PMID: 15600306
-
Differentiation of MC3T3-E1 on poly(4-styrenesulfonic acid-co-maleic acid)sodium salt-coated films.Dent Mater J. 2011;30(2):158-69. doi: 10.4012/dmj.2010-097. Epub 2011 Mar 16. Dent Mater J. 2011. PMID: 21422666
Cited by
-
Effect of Static Pre-stretch Induced Surface Anisotropy on Orientation of Mesenchymal Stem Cells.Cell Mol Bioeng. 2014 Mar 1;7(1):106-121. doi: 10.1007/s12195-013-0300-0. Cell Mol Bioeng. 2014. PMID: 24678348 Free PMC article.
-
Human coronary artery smooth muscle cell responses to bioactive polyelectrolyte multilayer interfaces.Biotechnol Res Int. 2011;2011:854068. doi: 10.4061/2011/854068. Epub 2010 Nov 29. Biotechnol Res Int. 2011. PMID: 21350669 Free PMC article.
-
Utilization of the Theory of Small on Large Deformation for Studying Mechanosensitive Cellular Behaviors.J Elast. 2019 Aug;136(2):137-157. Epub 2018 Sep 25. J Elast. 2019. PMID: 31598031 Free PMC article.
-
Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering.Chem Mater. 2012 Mar 13;24(5):854-869. doi: 10.1021/cm2032459. Chem Mater. 2012. PMID: 25076811 Free PMC article.
-
Noneluting enzymatic antibiofilm coatings.ACS Appl Mater Interfaces. 2012 Sep 26;4(9):4708-16. doi: 10.1021/am3010847. Epub 2012 Sep 4. ACS Appl Mater Interfaces. 2012. PMID: 22909396 Free PMC article.
References
-
- Kidambi S, Lee I, Chan C. Journal of the American Chemical Society. 2004;126:16286–16287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

