Stem cell models of cardiac development and disease
- PMID: 20604707
- PMCID: PMC3955884
- DOI: 10.1146/annurev-cellbio-100109-103948
Stem cell models of cardiac development and disease
Abstract
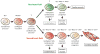
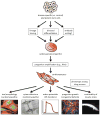
The past few years have witnessed remarkable advances in stem cell biology and human genetics, and we have arrived at an era in which patient-specific cell and tissue models are now practical. The recent identification of cardiovascular progenitor cells, as well as the identification of genetic variants underlying congenital heart disorders and adult disease, opens the door to the development of human models of human cardiovascular disease. We review the current understanding of the contribution of progenitor cells to cardiogenesis and outline how pluripotent stem cells can be applied to the modeling of cardiovascular disorders of genetic origin. A key challenge will be to implement these models in an efficient manner to develop a molecular understanding of how genes lead to disease and to screen for genes and drugs that modify the disease process.
Figures


References
-
- Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–27. - PubMed
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–8. - PubMed
-
- Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, et al. Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–5. - PubMed
-
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

