Enzymatic functions of wild tomato methylketone synthases 1 and 2
- PMID: 20605911
- PMCID: PMC2938155
- DOI: 10.1104/pp.110.157073
Enzymatic functions of wild tomato methylketone synthases 1 and 2
Abstract
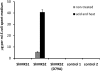
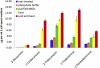
The trichomes of the wild tomato species Solanum habrochaites subsp. glabratum synthesize and store high levels of methylketones, primarily 2-tridecanone and 2-undecanone, that protect the plants against various herbivorous insects. Previously, we identified cDNAs encoding two proteins necessary for methylketone biosynthesis, designated methylketone synthase 1 (ShMKS1) and ShMKS2. Here, we report the isolation of genomic sequences encoding ShMKS1 and ShMKS2 as well as the homologous genes from the cultivated tomato, Solanum lycopersicum. We show that a full-length transcript of ShMKS2 encodes a protein that is localized in the plastids. By expressing ShMKS1 and ShMKS2 in Escherichia coli and analyzing the products formed, as well as by performing in vitro assays with both ShMKS1and ShMKS2, we conclude that ShMKS2 acts as a thioesterase hydrolyzing 3-ketoacyl-acyl carrier proteins (plastid-localized intermediates of fatty acid biosynthesis) to release 3-ketoacids and that ShMKS1 subsequently catalyzes the decarboxylation of these liberated 3-ketoacids, forming the methylketone products. Genes encoding proteins with high similarity to ShMKS2, a member of the "hot-dog fold" protein family that is known to include other thioesterases in nonplant organisms, are present in plant species outside the genus Solanum. We show that a related enzyme from Arabidopsis (Arabidopsis thaliana) also produces 3-ketoacids when recombinantly expressed in E. coli. Thus, the thioesterase activity of proteins in this family appears to be ancient. In contrast, the 3-ketoacid decarboxylase activity of ShMKS1, which belongs to the alpha/beta-hydrolase fold superfamily, appears to have emerged more recently, possibly within the genus Solanum.
Figures





References
-
- Antonious GF. (2001) Production and quantification of methyl ketones in wild tomato accessions. J Environ Sci Health B 36: 835–848 - PubMed
-
- Benning MM, Wesenberg G, Liu RQ, Taylor KL, Dunaway-Mariano D, Holden HM. (1998) The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. strain CBS-3. J Biol Chem 50: 33572–33579 - PubMed
-
- Buchanan BB, Gruissem W, Jones RL. (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Biology, Rockville, MD
-
- Forouhar F, Yang Y, Kumar D, Chen Y, Fridman E, Park SW, Chiang Y, Acton TB, Montelione GT, Pichersky E, et al. (2005) Identification of methyl salicylate as a substrate for salicylic acid binding protein 2 (SABP2) and implications for plant host defense. Proc Natl Acad Sci USA 102: 1773–1778 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

