The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites
- PMID: 20605978
- PMCID: PMC2937440
- DOI: 10.1128/IAI.00502-09
The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites
Abstract
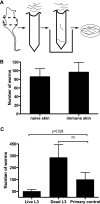
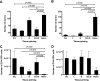
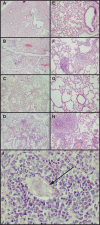
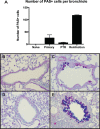
The rodent hookworm Nippostrongylus brasiliensis typically infects its host by penetrating the skin and rapidly migrating to the lungs and gut. Following primary infection, immunocompetent mice become highly protected from reinfection with N. brasiliensis, with the numbers of worms gaining access to the lungs and gut being reduced by up to 90%. We used green fluorescent protein/interleukin-4 (IL-4) reporter mice and truncated infection studies to identify both the tissue site and mechanism(s) by which the host protects itself from reinfection with N. brasiliensis. Strikingly, we demonstrated that the lung is an important site for priming immune protection. Furthermore, a lung-initiated, CD4 T-cell-dependent, and IL-4- and STAT6-dependent response was sufficient to confer protection against reinfection. In conclusion, vaccination strategies which seek to break the cycle of reinfection and egg production by helminths such as hookworms can include strategies which directly stimulate Th2 responses in the lung.
Figures


References
-
- Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. - PubMed
-
- Aitken, R., P. S. Coulson, and R. A. Wilson. 1988. Pulmonary leukocytic responses are linked to the acquired immunity of mice vaccinated with irradiated cercariae of Schistosoma mansoni. J. Immunol. 140:3573-3579. - PubMed
-
- Brown, A., N. Girod, E. E. Billett, and D. I. Pritchard. 1999. Necator americanus (human hookworm) aspartyl proteinases and digestion of skin macromolecules during skin penetration. Am. J. Trop. Med. Hyg. 60:840-847. - PubMed
-
- Camberis, M., G. Le Gros, and J. Urban, Jr. 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. Chapter 19:Unit 19.12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

